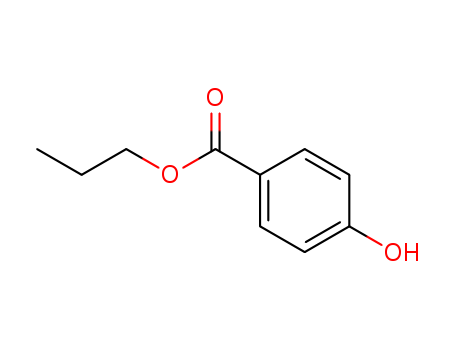
- Chemical Name:Propylparaben
- CAS No.:94-13-3
- Deprecated CAS:59593-07-6,58339-85-8,58339-85-8
- Molecular Formula:C10H12O3
- Molecular Weight:180.203
- Hs Code.:29182930
- European Community (EC) Number:202-307-7,920-388-3
- NSC Number:23515,8511
- UNII:Z8IX2SC1OH
- DSSTox Substance ID:DTXSID4022527
- Nikkaji Number:J3.943H
- Wikipedia:Propylparaben
- Wikidata:Q511627
- NCI Thesaurus Code:C76730
- RXCUI:34706
- Metabolomics Workbench ID:45619
- ChEMBL ID:CHEMBL194014
- Mol file:94-13-3.mol
Synonyms:3(or 4)-hydroxybenzoic acid propyl ester;4-hydroxybenzoic acid propyl ester;Bayer D 206;Nipazol;p-hydroxybenzoic acid propyl ester;PEPH;propyl 4-hydroxybenzoate;propyl p-hydroxybenzoate;propylparaben;propylparaben, monosodium salt


 Xi
Xi


