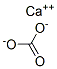
Suppliers and Price of Aragonite (Ca(CO3)) (9CI)
- Business phase:
- The product has achieved commercial mass production*data from LookChem market partment
- Manufacturers and distributors:
-
- Manufacture/Brand
- Chemicals and raw materials
- Packaging
- price
- Usbiological
- N-[3-[[4-
- 50mg
- $ 496.00
- TRC
- Calcium carbonate
- 100g
- $ 95.00
- TRC
- Calcium carbonate
- 50g
- $ 75.00
- Strem Chemicals
- Calcium carbonate, 98%
- 250g
- $ 64.00
- Strem Chemicals
- Calcium carbonate (99.95%-Ca)
- 100g
- $ 59.00
- Strem Chemicals
- Calcium carbonate (99.999%-Ca) PURATREM
- 10g
- $ 88.00
- Strem Chemicals
- Calcium carbonate (99.95%-Ca)
- 500g
- $ 235.00
- Strem Chemicals
- Calcium carbonate, 98%
- 1kg
- $ 190.00
- Strem Chemicals
- Calcium carbonate (99.999%-Ca) PURATREM
- 50g
- $ 349.00
- Sigma-Aldrich
- Calcium carbonate
precipitated, special grade (≤ 0,002% Fe) EMPROVE? ESSENTIAL Ph Eur,BP,USP
- 1020749450
- $ 9120.00
-
Total 882 raw suppliers


 Xi
Xi
