Base Information
Edit
- Chemical Name:Hydroxyapatite
- CAS No.:1306-06-5
- Deprecated CAS:12440-80-1,136841-77-5,196875-13-5,1059174-00-3,221359-86-0,205873-50-3,29796-40-5,1337-78-6,947383-76-8,1398505-50-4
- Molecular Formula:Ca5.(OH).(PO4)3
- Molecular Weight:502.31
- Hs Code.:28352600
- European Community (EC) Number:215-145-7,235-330-6
- UNII:91D9GV0Z28
- DSSTox Substance ID:DTXSID50872537
- Wikipedia:Hydroxyapatite,Hydroxylapatite
- Wikidata:Q27271399
- NCI Thesaurus Code:C84224
- ChEMBL ID:CHEMBL2218916
- Mol file:1306-06-5.mol
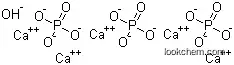
Synonyms:Alveograf;Calcitite;Calcium Hydroxyapatite;Durapatite;Hydroxyapatite;Hydroxyapatite, Calcium;Hydroxylapatite;Interpore 200;Interpore 500;Interpore-200;Interpore-500;Interpore200;Interpore500;Osprovit;Ossein Hydroxyapatite Compound;Ossein-Hydroxyapatite Compound;Ossopan;Osteogen;Periograf


 Xi
Xi
