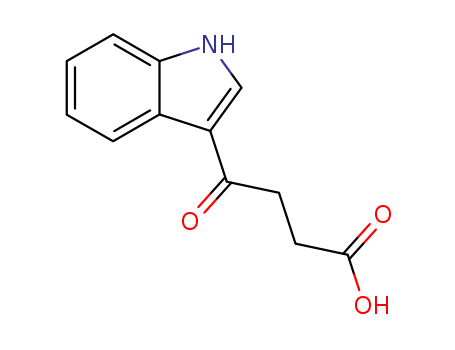
- Chemical Name:4-(1H-indol-3-yl)-4-oxobutanoic acid
- CAS No.:835-45-0
- Molecular Formula:C12H11NO3
- Molecular Weight:217.22
- Hs Code.:2933990090
- European Community (EC) Number:676-042-4
- DSSTox Substance ID:DTXSID60352597
- Nikkaji Number:J1.611.691B
- Wikidata:Q72498793
- ChEMBL ID:CHEMBL1477954
- Mol file:835-45-0.mol
Synonyms:4-(1H-indol-3-yl)-4-oxobutanoic acid;835-45-0;Indole-3-(4'-oxo)butyric acid;MLS000060927;4-(1H-Indol-3-yl)-4-oxo-butyric acid;4-(1h-Indol-3-yl)-4-oxobutanoicacid;SMR000069155;CBKinase1_000052;CBKinase1_012452;Cambridge id 5213202;TimTec1_003000;Oprea1_673990;Oprea1_737056;cid_730037;SCHEMBL7214385;CHEMBL1477954;DTXSID60352597;NLUOPJQCFYTMGC-UHFFFAOYSA-N;BDBM113995;HMS1542I08;HMS2392H18;indole-3-(4'-oxo) butyric acid;4-oxo-4-(3-indolyl)butyric acid;MFCD00559697;STK088693;AKOS000629536;SDCCGMLS-0006309.P002;AS-82222;4-(1H-indol-3-yl)-4-keto-butyric acid;CS-0171429;FT-0630306;AB00075416-01;4-(1H-indol-3-yl)-4-oxidanylidene-butanoic acid;SR-01000619555;Q-102927;SR-01000619555-2;(4-(1-(tert-butoxycarbonyl)-4-cyanopiperidin-4-yl)phenyl)boronic acid