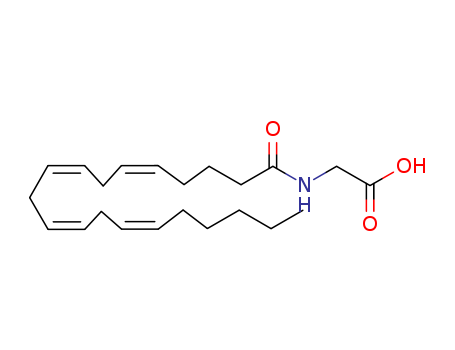
- Chemical Name:N-arachidonoylglycine
- CAS No.:179113-91-8
- Molecular Formula:C22H35NO3
- Molecular Weight:361.52
- Hs Code.:
- NSC Number:746569
- UNII:5PB56SQA6E
- DSSTox Substance ID:DTXSID5045124
- Nikkaji Number:J1.404.839A
- Wikipedia:N-Arachidonylglycine
- Wikidata:Q18378715
- Pharos Ligand ID:TTGWLUPKZJZF
- Metabolomics Workbench ID:4504
- ChEMBL ID:CHEMBL161343
- Mol file:179113-91-8.mol
Synonyms:EMA-1 20:4;N-arachidonoyl glycine;N-arachidonylglycine;NAGly amino acid