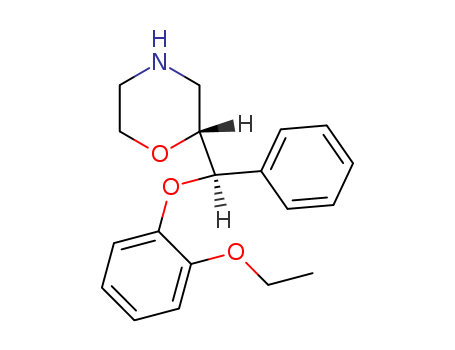
- Chemical Name:Esreboxetine
- CAS No.:98819-76-2
- Molecular Formula:C19H23NO3
- Molecular Weight:313.397
- Hs Code.:
- UNII:L8S50ZY490
- DSSTox Substance ID:DTXSID601009938
- Nikkaji Number:J569.656I
- Wikipedia:Esreboxetine
- Wikidata:Q5399299
- NCI Thesaurus Code:C175719
- Pharos Ligand ID:C7VY6F2T6RYT
- Metabolomics Workbench ID:42636
- ChEMBL ID:CHEMBL180101
- Mol file:98819-76-2.mol
Synonyms:(+)-(2S)-2-((S)-(2-ethoxyphenoxy)phenylmethyl)morpholine;esreboxetine