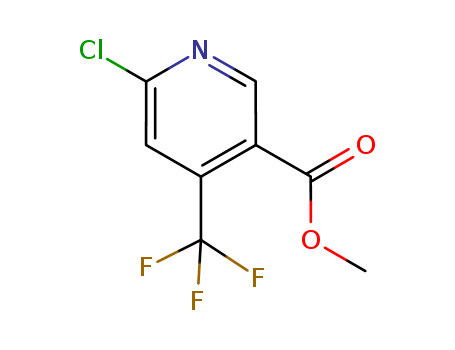
- Chemical Name:Methyl 6-chloro-4-(trifluoromethyl)nicotinate
- CAS No.:261635-79-4
- Molecular Formula:C8H5ClF3NO2
- Molecular Weight:239.581
- Hs Code.:2933399090
- European Community (EC) Number:671-232-3
- DSSTox Substance ID:DTXSID10379532
- Wikidata:Q82169382
- Mol file:261635-79-4.mol
Synonyms:METHYL 6-CHLORO-4-(TRIFLUOROMETHYL)NICOTINATE;261635-79-4;methyl 6-chloro-4-(trifluoromethyl)pyridine-3-carboxylate;MFCD01941315;3-Pyridinecarboxylic acid, 6-chloro-4-(trifluoromethyl)-, methyl ester;SCHEMBL473983;DTXSID10379532;BBL102191;STL555990;AKOS015851235;PS-11258;methyl 6-chloro-4-trifluoromethylnicotinate;CS-0046073;FT-0648513;Methyl6-chloro-4-(trifluoromethyl)nicotinate;methyl-6-chloro-4-(trifluoromethyl)nicotinate;C74354;EN300-133309;methyl 6-chloro-4-(trifluoromethyl)-nicotinate;methyl-6-chloro-4-(trifluoromethyl)-nicotinate;6-chloro-4-trifluoromethyl-nicotinic acid methyl ester;methyl 6-chloro-4-trifluoromethylpyridin-3-ylcarboxylate;Z1509716046;Methyl 6-chloro-4-(trifluoromethyl)nicotinate, AldrichCPR



 Xi
Xi


