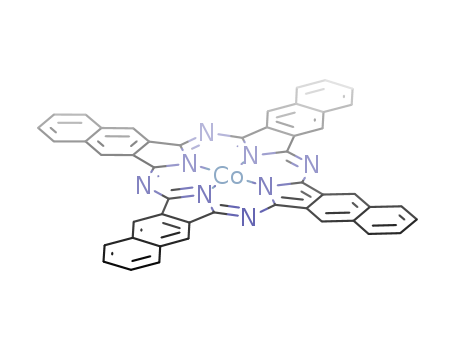
- Chemical Name:Cobalt(ii)2,3-naphthalocyanine
- CAS No.:26603-20-3
- Molecular Formula:C48H24CoN8
- Molecular Weight:771.765
- Hs Code.:
- European Community (EC) Number:622-588-3
- Mol file:26603-20-3.mol
Synonyms:Cobalt(ii)2,3-naphthalocyanine;Cobalt(II) 2,3-naphthalocyanine;26603-20-3



 Xn
Xn


