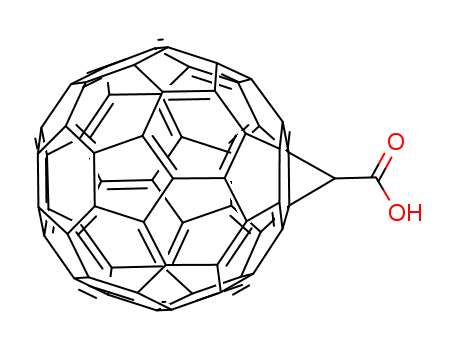
- Chemical Name:(1,2-METHANOFULLERENE C60)-61-CARBOXYLIC ACID
- CAS No.:155116-19-1
- Molecular Formula:C62H2 O2
- Molecular Weight:778.697
- Hs Code.:
- Mol file:155116-19-1.mol
Synonyms:(1,2-METHANOFULLERENE C60)-61-CARBOXYLIC ACID;(1,2-METHANOFULLERENE C(60))-61-CARBOXY;(1 2-METHANOFULLERENE C(60))-61-CARBO- &