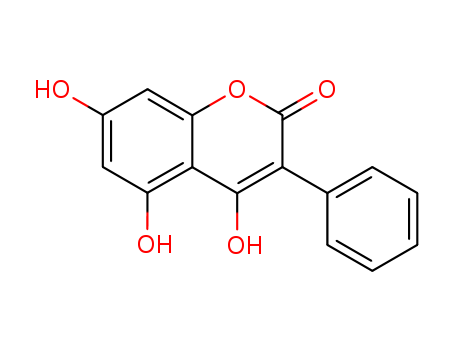
- Chemical Name:4,5,7-TRIHYDROXY-3-PHENYLCOUMARIN
- CAS No.:4222-02-0
- Molecular Formula:C15H10O5
- Molecular Weight:270.241
- Hs Code.:2932209090
- Mol file:4222-02-0.mol
Synonyms:Coumarin,4,5,7-trihydroxy-3-phenyl- (6CI,7CI,8CI);3-Phenyl-4,5,7-trihydroxycoumarin;4,5,7-Trihydroxy-3-phenylcoumarin;



 Xi
Xi


