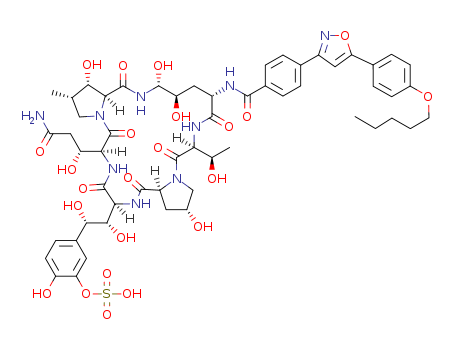
- Chemical Name:Micafungin
- CAS No.:235114-32-6
- Molecular Formula:C56H71N9O23S
- Molecular Weight:1270.29
- Hs Code.:
- Mol file:235114-32-6.mol
Synonyms:Mycamine;1H-dipyrrolo[2,1-c:2',1'-l][1,4,7,10,13,16]hexaazacycloheneicosine-20-propanamide, 23-[1,2-dihydroxy-2-[4-hydroxy-3-(sulfooxy)phenyl]ethyl]tetracosahydro-β,2,11,12,15-pentahydroxy-6-(1-hydroxyethyl)-16-methyl-5,8,14,19,22,25-hexaoxo-9-[[4-[5-[4-(pentyloxy)phenyl]-3-isoxazolyl]benzoyl]amino]-;