- Chemical Name:p-Terphenyl-4-carboxylic acid
- CAS No.:5731-15-7
- Molecular Formula:C19H14O2
- Molecular Weight:274.31326
- Hs Code.:
- European Community (EC) Number:624-104-6
- DSSTox Substance ID:DTXSID80433493
- Nikkaji Number:J2.053.805H
- Wikidata:Q82247653
- Mol file:5731-15-7.mol
Synonyms:p-Terphenyl-4-carboxylic acid;5731-15-7;4-(4-phenylphenyl)benzoic Acid;[1,1':4',1''-Terphenyl]-4-carboxylicacid;[1,1':4',1''-Terphenyl]-4-carboxylic acid;SCHEMBL7155730;DTXSID80433493;p-Terphenyl-4-carboxylic acid, 97%;AKOS009041385;CS-0181275;1,1':4',1''-Terbenzene-4-carboxylic acid



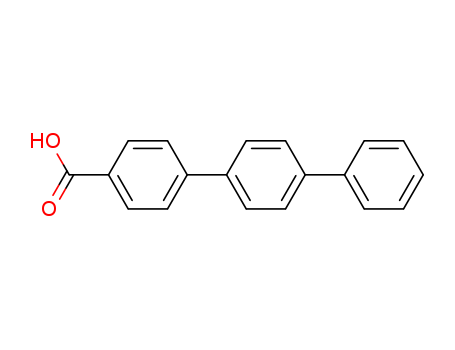
 Xn,
Xn, N
N


