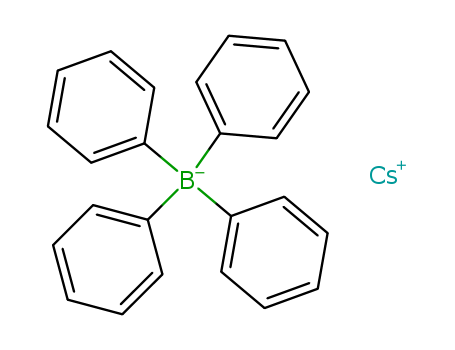
- Chemical Name:CESIUM TETRAPHENYLBORATE
- CAS No.:3087-82-9
- Molecular Formula:C24H20 B . Cs
- Molecular Weight:452.139
- Hs Code.:2931900090
- Mol file:3087-82-9.mol
Synonyms:Borate(1-),tetraphenyl-, cesium (8CI,9CI); Cesium tetraphenylborate (7CI); Cesiumtetraphenylborate(1-)