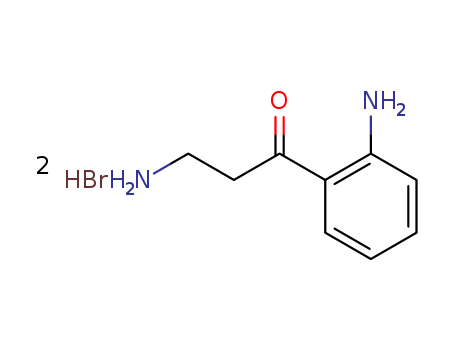
- Chemical Name:KYNURAMINE DIHYDROBROMIDE
- CAS No.:304-47-2
- Molecular Formula:2BrH*C9H12N2O
- Molecular Weight:326.031
- Hs Code.:
- Mol file:304-47-2.mol
Synonyms:1-Propanone,3-amino-1-(2-aminophenyl)-, dihydrobromide (9CI); Propiophenone, 2',3-diamino-,dihydrobromide (7CI,8CI); Kynuramine dihydrobromide