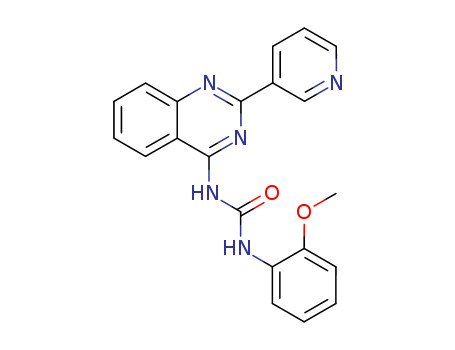
- Chemical Name:1-(2-Methoxyphenyl)-3-(2-pyridin-3-ylquinazolin-4-yl)urea
- CAS No.:280570-45-8
- Molecular Formula:C21H17N5O2
- Molecular Weight:371.398
- Hs Code.:
- DSSTox Substance ID:DTXSID00398737
- Nikkaji Number:J1.372.036C
- Wikidata:Q27089203
- Pharos Ligand ID:A71DAD7RN3X6
- ChEMBL ID:CHEMBL70880
- Mol file:280570-45-8.mol
Synonyms:VUF 5574;280570-45-8;VUF5574;VUF-5574;1-(2-methoxyphenyl)-3-(2-pyridin-3-ylquinazolin-4-yl)urea;CHEMBL70880;3-(2-methoxyphenyl)-1-(2-pyridin-3-ylquinazolin-4-yl)urea;1-(2-Methoxyphenyl)-3-(2-(pyridin-3-yl)quinazolin-4-yl)urea;NCGC00016084-01;Lopac-V-5888;D01AAI;Lopac0_001247;SCHEMBL935642;GTPL3280;DTXSID00398737;YRAFEJSZTVWUMD-UHFFFAOYSA-N;BDBM50088475;AKOS000749577;CCG-205321;NCGC00016084-02;NCGC00016084-03;NCGC00016084-04;NCGC00016084-05;NCGC00094488-01;NCGC00094488-02;NCGC00094488-03;NCGC00094488-04;MS-25980;HY-103189;CS-0025280;EU-0101247;EC-000.2112;V 5888;SR-01000076193;J-016958;SR-01000076193-1;Q27089203;1-(2-Methoxy-phenyl)-3-(2-pyridin-3-yl-quinazolin-4-yl)-urea;N-(2-Methoxyphenyl)-N'-[2-(3-pyridinyl)-4-quinazolinyl]-urea;N-(2-Methoxyphenyl)-N'-[2-(3-pyrindinyl)-4-quinazolinyl]-urea