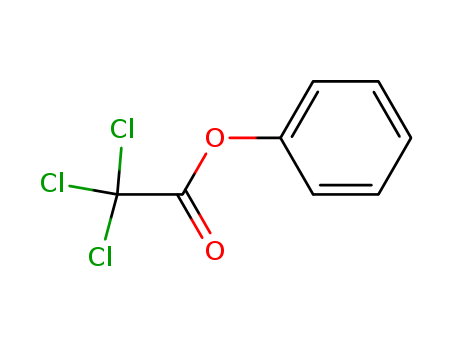
- Chemical Name:Phenyl trichloroacetate
- CAS No.:10112-13-7
- Molecular Formula:C8H5 Cl3 O2
- Molecular Weight:239.485
- Hs Code.:2915400090
- NSC Number:141459
- DSSTox Substance ID:DTXSID10301156
- Nikkaji Number:J1.809.204B
- Wikidata:Q82044788
- Mol file:10112-13-7.mol
Synonyms:Phenyl trichloroacetate;phenyl 2,2,2-trichloroacetate;10112-13-7;Phenyl trichloroacetate #;SCHEMBL987567;DTXSID10301156;Trichloroacetic acid, phenyl ester;NSC141459;NSC-141459;phenyl 2,2,2-tris(chloranyl)ethanoate;2,2,2-trichloroacetic acid phenyl ester;A841510