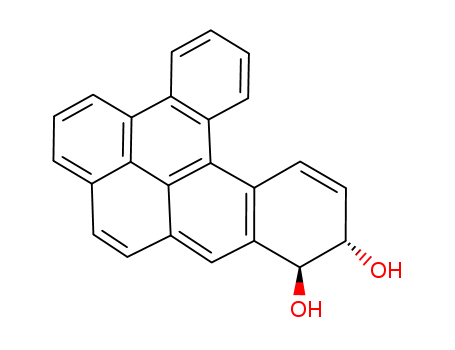
- Chemical Name:Dibenzo[a,def]triphenylene-11,12-diol,11,12-dihydro-, (11R,12R)-
- CAS No.:189880-63-5
- Molecular Formula:C24H16O2
- Molecular Weight:336.39
- Hs Code.:
- Mol file:189880-63-5.mol
Synonyms:Dibenzo[def,p]chrysene-11,12-diol,11,12-dihydro-, (11R,12R)- (9CI); Dibenzo[def,p]chrysene-11,12-diol,11,12-dihydro-, (11R-trans)-;(-)-(R,R)-11,12-Dihydroxy-11,12-dihydrodibenzo[a,l]pyrene;(-)-(R,R)-trans-Dibenzo[a,l]pyrene-11,12-dihydrodiol;(-)-trans-11R,12R-Dibenzo[a,l]pyrenedihydrodiol