|
Name
|
|
EINECS
|
215-226-7
|
|
CAS No.
|
1314-22-3
|
Density
|
1.57 g/mL at 25 °C(lit.)
|
|
PSA
|
46.12000
|
LogP
|
-0.24010
|
|
Solubility
|
Insoluble in water.
|
Melting Point
|
212 °C (dec.)(lit.)
|
|
Formula
|
ZnO2
|
Boiling Point
|
150.2 °C at 760 mmHg
|
|
Molecular Weight
|
97.3888
|
Flash Point
|
N/A
|
|
Transport Information
|
UN 1516 5.1/PG 2
|
Appearance
|
white powder
|
|
Safety
|
24/25-27-17-61-60
|
Risk Codes
|
9-50/53-8
|
|
Molecular Structure
|
|
Hazard Symbols
|
 O O
|
|
Synonyms
|
Struktol ZP1014;ZP 1014;ZPO;Zeonet ZP;Zinc dioxide;Zinc oxide (ZnO2);
|
Article Data |
12
|
ZINC PEROXIDE Synthetic route
ZINC PEROXIDE Chemical Properties
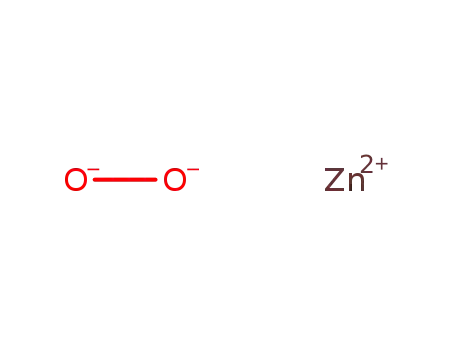
The molecular structure of Zinc peroxide (1314-22-3) as follow: 
EINECS: 215-226-7
Molecular formula: O2Zn
Formula weight: 97.39
Synonyms: Zincperoxide(Zn(O2)) ; Zincperoxide,medicinal ; Zinc dioxide
Density: 1.57 g/mL at 25 ℃(lit.)
Melting point: 212 ℃ (dec.)(lit.)
Boiling point: 150.2 ℃ at 760 mmHg
Vapour pressure: 1.48 mmHg at 25 ℃
Appearance: White to light yellow odorless powder
Zinc peroxide (1314-22-3) is insoluble in water but can be decomposed slowly by water. It has cubic crystalline structure which is the same as FeS2 , a bulk modulus of 174 GPa, and it is paramagnetic down to 5 K. The product categories about it belong to inorganics; peroxides chemical synthesis; catalysis and inorganic chemistry; oxidation; oxides; synthetic reagents; zinc; zinc metal and ceramic science.
ZINC PEROXIDE Uses
Zinc peroxide (1314-22-3) is used as rubber accelerator, curing agent for synthetic elastomers and bleaching agent. It can be applied in pharmaceuticals. Another application of Zinc peroxide (1314-22-3) is additive to antiseptic ointments and it has also been used as an oxidant in explosives and pyrotechnic mixtures.
ZINC PEROXIDE Toxicity Data With Reference
Zinc peroxide (1314-22-3) is not listed as a carcinogen by ACGIH, IARC, NTP, or CA Prop 65 and the toxicological properties have not been fully investigated.
ZINC PEROXIDE Consensus Reports
Zinc and its compounds are on the Community Right-To-Know List. Reported in EPA TSCA Inventory.
ZINC PEROXIDE Safety Profile
Systemic toxicity is similar to zinc oxide. Flammable when exposed to heat or by chemical reaction with reducing materials. Finely divided powder is slightly soluble in water, decomposes rapidly at 150 ℃. A powerful oxidizer and dangerous when mixed with highly combustible materials. A very dangerous explosion hazard when exposed to heat. Very dangerous, will react with water or steam to produce heat. Vigorous reaction with reducing materials. When heated to decomposition it emits toxic fumes of ZnO. See also PEROXIDES, INORGANIC; PEROXIDES, ORGANIC and ZINC COMPOUNDS.
Hazard codes:  O Oxidizing
O Oxidizing  N Dangerous for the environment
N Dangerous for the environment
Risk statements: 8 Contact with combustible material may cause fire.
9 Explosive when mixed with combustible material.
50/53 Very Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment.
Safety statements: 17 Keep away from combustible material.
24/25 Avoid contact with skin and eyes.
27 Take off immediately all contaminated clothing.
60 This material and/or its container must be disposed of as hazardous waste.
61 Avoid release to the environment. Refer to special instructions safety data sheet.
ZINC PEROXIDE Standards and Recommendations
DOT Classification: 5.1; Label: Oxidizer
ZINC PEROXIDE Specification
Zinc peroxide (1314-22-3) dissolves in dilute acid, liberating hydrogen peroxide. It is noncombustible but accelerates the burning of combustible material. If the combustible material is divided finely, the mixture may be explosive. Sometimes it can be ignited by friction or contact with moisture in case it mixtures with combustible material. The hydrated material (of indefinite composition) explodes at 212 ℃. It could burn brilliantly if it mixtures with aluminum or zinc powder. It might produce irritating and/or toxic gases in case of fire.
Keep Zinc peroxide (1314-22-3) in a cool, dry, dark location in a tightly sealed container or cylinder. Keep it away from incompatible materials, combustible materials and heat.





 O
O













 O Oxidizing
O Oxidizing  N Dangerous for the environment
N Dangerous for the environment 

