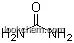Products Categories
| CAS No.: | 124-43-6 |
|---|---|
| Name: | Urea hydrogen peroxide |
| Article Data: | 262 |
| Molecular Structure: | |
|
|
|
| Formula: | CH6N2O3 |
| Molecular Weight: | 94.0702 |
| Synonyms: | Hydrogen peroxide-urea compound (1:1);Percarbamide;Urea,compounds,compd. with hydrogen peroxide (H2O2) (1:1);Perhydrol-Urea;Percarbamid;Carbamide peroxide, solution;Urea hydroperoxide;Gly-oxide;Ortizon;Hydrogen peroxide, compd. with urea (1:1);Carbamide peroxide;Thenardol;hydrogen peroxide; urea;Perhydrit;Hydroperit;Hydroperite;Urea, compd. with hydrogen peroxide (H2O2) (1:1);Urea dioxide;Urea Peroxide; |
| EINECS: | 204-701-4 |
| Melting Point: | 90-93 °C(lit.) |
| Boiling Point: | 196.6 °C at 760 mmHg |
| Flash Point: | 72.7 °C |
| Solubility: | water: 0.5 g/mL, clear to very faintly hazy |
| Appearance: | white crystals or powder |
| Hazard Symbols: |
 Xi, Xi, C, C, O O
|
| Risk Codes: | 8-34-36/37/38 |
| Safety: | 26-36-45-36/37/39-17 |
| Transport Information: | UN 1511 5.1/PG 3 |
| PSA: | 109.57000 |
| LogP: | 0.44180 |
- 144851-82-1METHYL2-AMINO-3-FLUOROBENZOATE
- 483366-12-7(2S,4R)-1-Boc-2-cyano-4-hydroxypyrrolidine
- 173606-50-3BOC-10-AMINODECANOIC ACID
- 361456-36-2METHYL (R)-(+)-ISOCYANATO-3-PHENYLPROPI&
- 5156-58-1N-(1-Benzyl-4-pipperidinyl)-N-phenylpropanamide HCl
- 81281-59-67-Benzylideneaminotheophylline
- 50288-62-5threo-Phenyl-2-piperidyl acetamide
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 47087-37-6Z-D-Glu-OMe
- 73441-42-6METHYL-5-CHLORO-2,2-DIMETHYLVALERATE

| Conditions | Yield |
|---|---|
| Stage #1: sodium molybdate; ammonium dihydrogen phosphate With caesium carbonate In neat (no solvent, solid phase) at 850℃; for 24h; Stage #2: In neat (no solvent, solid phase) at 680℃; for 85h; Stage #3: In neat (no solvent, solid phase) at 20℃; for 66h; |

| Conditions | Yield |
|---|---|
| Stage #1: ammonium dihydrogen phosphate; sodium carbonate In neat (no solvent, solid phase) at 350℃; for 24h; Stage #2: In neat (no solvent, solid phase) at 600℃; for 120h; Calcination; |

- 124-43-6
sodium pyrophosphate

| Conditions | Yield |
|---|---|
| With silver cyanide In neat (no solvent) byproducts: NaCN, NaCNO, Ag; heating with AgCN in presence of (CN)2 to 270°C;; | 75% |
| With sulfur In neat (no solvent) grinding phosphate with sulfur; heating to 1000°C in quartz crucible in a stream of air (10 K/min);; detn. by 31P-NMR; | |
| With mercury(II) cyanide In neat (no solvent) molar ratio Na3PO4:Hg(CN)2=1:1 or 2:1, heating for 1.5h;; | 74-77 |
| With oxygen; Nitrogen dioxide In neat (no solvent) Kinetics; byproducts: NaNO3; mixing with NO2 and O2 directly in the quartz reaction vessel contg. Na3PO4, heating on a platinum support at different temp. for different times; not isolated; Raman, NMR; |

- 124-43-6
sodium pyrophosphate

| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: H2O; heating to 300°C for several days;; | |
| byproducts: H2O; heating at 100-105°C to const. mass; | |
| In solid byproducts: H2O; dehydrating the decahydrate at 130°C to constant weight; |

- 124-43-6
sodium pyrophosphate

| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: H2O; dehydration at elevated temp., decompn. at red heat (Pt-vessel);; | |
| In melt; neat (no solvent) byproducts: H2O; dehydration of melt in presence of 2-25% condensed phosphates; heating resulting dihydrate to 300-400°C;; | |
| In melt; neat (no solvent) byproducts: H2O; dehydration of melt, heating resulting dihydrate to 300-400°C;; |

| Conditions | Yield |
|---|---|
| With H2O In water formation of orthophosphate and pyrophosphate by hydratation of metaphosphate in alkaline solns.;; |

- 124-43-6
sodium pyrophosphate

| Conditions | Yield |
|---|---|
| sodium nitrate In neat (no solvent) increase of react. rate by NaNO3 (decompn. of impurities);; | |
| With sodium nitrate In neat (no solvent) oxidation of organic material in educt mixt. by NaNO3, light product;; | |
| With thionyl chloride In neat (no solvent) byproducts: SO2, HCl; heating on a water bath;; |

- 124-43-6
sodium pyrophosphate

| Conditions | Yield |
|---|---|
| In melt thermal decompn.;; | |
| With water In water byproducts: HF; hydrolyzed at 883 K; | |
| In neat (no solvent) on heating;; | |
| In neat (no solvent) on heating;; |

- 124-43-6
sodium pyrophosphate

| Conditions | Yield |
|---|---|
| With air In neat (no solvent) spraying Na posphates (Na2O:P2O5 >2:1) into a P flame, temp. up to 1000°C;; | |
| With air In sodium hydroxide P flame, react. vessel coated with a layer of alk. orthophosphate-soln. (Na2O:P2O5=2:1), dehydration of resulting soln., calcination to Na4P2O7;; | |
| With air In neat (no solvent) spraying sodium-phosphates-hydrate melts (Na2O:P2O5 >2:1) into a P flame, temp. up to 1000°C;; |

- 124-43-6
sodium pyrophosphate

| Conditions | Yield |
|---|---|
| With oxygen In neat (no solvent) Kinetics; on heating;; | |
| With sodium nitrite In neat (no solvent) 240-570°C, different molar ratios of Na2O and P2O5;; | |
| With NaNO2 In neat (no solvent) 240-570°C, different molar ratios of Na2O and P2O5;; | |
| With O2; Na-acetate or NaNO3 or Na2CO3 In neat (no solvent) Kinetics; on heating;; |
 This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
Standards and Recommendations
Specification
The Urea hydrogen peroxide,with the CAS registry number 124-43-6, it is also named as carbamide peroxide. The Hydrogen peroxide with urea (1:1) is an organic compound with the formula CH6N2O3. The IUPAC name of this chemical is hydrogen peroxide; urea. The product's classification code is Anti-infective, topical [dental]. Besides, it is a white crystals or powder, which should be stored in a dry and well-ventilated place at temperature of 15 - 25 °C. In medicine and the pharmaceutical industry, it can be used as an efficient safe and convenient solid disinfectant.this chemical can be prepared by low concentrations of hydrogen peroxide and saturated or supersaturated urea solution.
Physical properties about Urea hydrogen peroxide with urea (1:1) are:
(1)ACD/LogP: -2.11; (2)ACD/LogD (pH 5.5): -2.11; (3)ACD/LogD (pH 7.4): -2.11; (4)ACD/BCF (pH 5.5): 1; (5)ACD/BCF (pH 7.4): 1; (6)ACD/KOC (pH 5.5): 1.69; (7)ACD/KOC (pH 7.4): 1.69; (8)#H bond acceptors: 3; (9)#H bond donors: 4; (10)Polar Surface Area: 23.55 Å2; (11)Flash Point: 72.7 °C; (12)Enthalpy of Vaporization: 43.28 kJ/mol; (13)Boiling Point: 196.6 °C at 760 mmHg; (14)Vapour Pressure: 0.395 mmHg at 25°C.
Safety of Urea hydrogen peroxide:
When you are using this chemical, please be cautious about it as the following: It contacts with combustible material may cause fire and can cause burns. Please keep away from combustible material. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. Besides, this chemical is irritating to eyes, respiratory system and skin. When you are using it, wear suitable gloves and eye/face protection. In case of accident or if you feel unwell seek medical advice immediately (show the label where possible).
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(N)N.OO
(2)InChI: InChI=1/CH4N2O.H2O2/c2-1(3)4;1-2/h(H4,2,3,4);1-2H
(3)InChIKey: AQLJVWUFPCUVLO-UHFFFAOYAW
(4)Std. InChI: InChI=1S/CH4N2O.H2O2/c2-1(3)4;1-2/h(H4,2,3,4);1-2H
(5)Std. InChIKey: AQLJVWUFPCUVLO-UHFFFAOYSA-N