Products Categories
Basic Information
| CAS No.: | 506-77-4 |
|---|---|
| Name: | Cyanogen chloride |
| Article Data: | 239 |
| Molecular Structure: | |
|
|
|
| Formula: | CCl N |
| Molecular Weight: | 61.4707 |
| Synonyms: | Cyanogenchloride (8CI); Chlorine cyanide; Chlorine cyanide (ClCN); Chlorocyan;Chlorocyanide; Chlorocyanide (ClCN); Chlorocyanogen; Cyanochloride (CNCl);Cyanogen chloride (ClCN) |
| EINECS: | 208-052-8 |
| Density: | 1.234 g/cm3 |
| Melting Point: | 6.5ºC |
| Boiling Point: | 13.8 °C at 760 mmHg |
| Flash Point: | °C |
| Solubility: | soluble in water, alcohol, ether |
| Appearance: | Colorless, liquid /gas |
| Hazard Symbols: |

|
| Risk Codes: | T:Toxic; ">  T:Toxic; T:Toxic; |
| Transport Information: | UN 1589 |
| PSA: | 23.79000 |
| LogP: | 0.70628 |
Related products
- 5156-58-1N-(1-Benzyl-4-pipperidinyl)-N-phenylpropanamide HCl
- 81281-59-67-Benzylideneaminotheophylline
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 73441-42-6METHYL-5-CHLORO-2,2-DIMETHYLVALERATE
- 68439-39-4Poly(oxy-1,2-ethanediyl), alpha-(2-ethylhexyl)-omega-hydroxy-,
- 852475-26-4MC1568
- 958254-66-51H-Imidazo[4,5-b]pyridine-2-carboxaldehyde, 1-methyl-, hydrochloride
- 99170-93-1N-Methyl-2-oxazolamine
- 717878-06-31-(4-fluorophenyl)-4-nitro-1H-imidazole
- 914458-26-7[5-(2-fluorophenyl)-1-pentyl-1H-pyrrol-3-yl]-1-naphthalenyl-Methanone
Synthetic route
Conditions

| Conditions | Yield |
|---|---|
| With thionyl chloride at 30 - 40℃; Product distribution; oth. reagent (SO2Cl2); | A 100% B 45% C n/a |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| With chlorine In water (CN)2/Cl2 = 1:1, at 560°C, in 10% CaCl2 soln.; cool and crystn. at -10°C; | 100% |
| With chlorine In gas at 900K; | 93% |
| With chlorine In gas at 1100K; | 89% |
- 773837-37-9
sodium cyanide

- 506-77-4
cyanogen chloride
Conditions

| Conditions | Yield |
|---|---|
| With chlorine In water continuous method, 15% NaCN soln.; | 98.4% |
| With chlorine In neat (no solvent) alkali cyanides, liquid Cl2 mixed in autoclave at -30°C; | 95% |
| With chlorine In hydrogenchloride at 0°C; distn.; | 90% |

- 506-77-4
cyanogen chloride
Conditions

| Conditions | Yield |
|---|---|
| With chlorine In water byproducts: KCl, ZnCl2; in suspension, 60-90 min. at room temp.; drying above CaCl2 and freezing with ice/NaCl or CO2/acetone mixt.; >99.9% pure; | 98% |
| With chlorine In not given byproducts: ZnCl2, KCl; at 0-5°C; product sepd. below 45°C; | |
| With Cl2 In not given byproducts: ZnCl2, KCl; at 0-5°C; product sepd. below 45°C; | |
| With Cl2 In not given byproducts: ZnCl2, KCl; at 0-5°C; product sepd. below 45°C; |

- 506-77-4
cyanogen chloride
Conditions

| Conditions | Yield |
|---|---|
| With chlorine In water byproducts: NaCl, ZnCl2; in suspension, 60-90 min. at room temp.; drying above CaCl2 and freezing with ice/NaCl or CO2/acetone mixt.; >99.9% pure; | 98% |
| With chlorine In not given byproducts: ZnCl2, NaCl; at 0-5°C; product sepd. below 45°; | |
| With Cl2 In not given byproducts: ZnCl2, NaCl; at 0-5°C; product sepd. below 45°; | |
| With Cl2 In not given byproducts: ZnCl2, NaCl; at 0-5°C; product sepd. below 45°; |

- 506-77-4
cyanogen chloride
Conditions

| Conditions | Yield |
|---|---|
| With chlorine In water byproducts: CaCl2, ZnCl2; in suspension, 60-90 min. at room temp.; drying above CaCl2 and freezing with ice/NaCl or CO2/acetone mixt.; >99.9% pure; | 98% |
| With chlorine In not given byproducts: ZnCl2, CaCl2; at 0-5°C; product sepd. below 45°C; | |
| With Cl2 In not given byproducts: ZnCl2, CaCl2; at 0-5°C; product sepd. below 45°C; | |
| With Cl2 In not given byproducts: ZnCl2, CaCl2; at 0-5°C; product sepd. below 45°C; |
Conditions

| Conditions | Yield |
|---|---|
| With chlorine In tetrachloromethane with alkali cyanides, under 3°C; | 98% |
| With chlorine In neat (no solvent) alkali cyanides, at -18°C; | 85% |
| With chlorine In neat (no solvent) alkali cyanides mixed with sand at 3°C; | 80% |
| With chlorine In tetrachloromethane alkali cyanides, at 0°C; | |
| With hydrogenchloride In not given |
Conditions

| Conditions | Yield |
|---|---|
| In neat (no solvent) in closed U-tube, at room temp., AgCN free from water; fract. distn. at -78°C; pure prod.; | 95% |
Conditions

| Conditions | Yield |
|---|---|
| With hydrogenchloride; dihydrogen peroxide; iron(III) In water at 50-65°C, continuous method; | 90% |
| With hydrogenchloride; dihydrogen peroxide; copper(II) ion In water at 50-65°C, continuous method; | 90% |
| With dihydrogen peroxide In water at 30°C; | 15% |
Conditions

| Conditions | Yield |
|---|---|
| With chlorine In hydrogenchloride alkali cyanides, pH=9; | A n/a B 90% |
 This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
Chemistry
Cyanogen chloride(506-77-4) is linear, triatomic pseudohalogen. Carbon and chlorine are linked by a single bond, and carbon and nitrogen by a triple bond.
Molecular Formula: CClN.
Molecular Structure: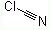 .
.
Molecular Weight: 61.47.
Density: 1.234 g/cm3.
Melting Point: 6.5 ℃.
Boiling Point: 13.8 ℃ at 760 mmHg.
Solubility: soluble in water, alcohol, ether.
Appearance: Colorless, liquid /gas.
Molecular Formula: CClN.
Molecular Structure:
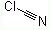 .
.Molecular Weight: 61.47.
Density: 1.234 g/cm3.
Melting Point: 6.5 ℃.
Boiling Point: 13.8 ℃ at 760 mmHg.
Solubility: soluble in water, alcohol, ether.
Appearance: Colorless, liquid /gas.
Uses
Cyanogen chloride(506-77-4) is widely used in biochemical analysis and preparation. Cyanogen chloride(506-77-4) is a common useful reagent in organic synthesis.For example, It is a precursor to the sulfonyl cyanides and chlorosulfonyl isocyanate.
Production
Cyanogen chloride(506-77-4) is produced by the oxidation of sodium cyanide with chlorine. This reaction proceeds via the intermediate cyanogen ((CN)2).
NaCN + Cl2 → ClCN + NaCl .
NaCN + Cl2 → ClCN + NaCl .
Toxicity Data With Reference
| 1. | eye-hmn 100 mg/m3/2M SEV | BJEPA5 British Journal of Experimental Pathology. 33 (1946),241. | ||
| 2. | ihl-hmn TCLo:10 mg/m3:EYE | WHOTAC World Health Organization, Technical Report Series. (1211 Geneva, 27, Switzerland) | ||
| 3. | ihl-man TCLo:2 g/m3:SKN | NTIS** National Technical Information Service. (Springfield, VA 22161) (Formerly U.S. Clearinghouse for Scientific and Technical Information) PB158-508 . | ||
| 4. | ihl-rat LC50:5400 mg/m3/3M | NTIS** National Technical Information Service. (Springfield, VA 22161) (Formerly U.S. Clearinghouse for Scientific and Technical Information) PB158-508 . | ||
| 5. | ihl-mus LC50:3 g/m3/30S | NTIS** National Technical Information Service. (Springfield, VA 22161) (Formerly U.S. Clearinghouse for Scientific and Technical Information) PB158-508 . | ||
| 6. | scu-mus LDLo:39 mg/kg | 27ZWAY Heffters Handbuch der Experimentelle Pharmakologie. | ||
| 7. | ihl-dog LC50:3800 mg/m3/1M | NTIS** National Technical Information Service. (Springfield, VA 22161) (Formerly U.S. Clearinghouse for Scientific and Technical Information) PB158-508 . | ||
| 8. | scu-dog LDLo:5 mg/kg | HBAMAK Abdernaldens Handbuch der Biologischen Arbeitsmethoden. 4 (1935),1341. | ||
| 9. | ihl-mky LC50:4400 mg/m3/1M | NTIS** |
Consensus Reports
Cyanide and its compounds are on the Community Right-To-Know List. Reported in EPA TSCA Inventory.
Safety Profile
Poison by ingestion, subcutaneous, and possibly other routes. Toxic by inhalation. Human systemic effects by inhalation: lachrymation, conjunctiva irritation, and chronic pulmonary edema or congestion. A primary irritant. A severe human eye irritant. An insecticide. Flammable when exposed to heat or flame. When heated to decomposition or on contact with water or steam, it will react to produce highly toxic and corrosive fumes of Cl−, CN−, and NOx. See also other cyanogen entries, CYANIDE, and CHLORIDES.
Standards and Recommendations
OSHA PEL: CL 0.3 ppm
ACGIH TLV: CL 0.3 ppm
DOT Classification: 2.3; Label: Poison Gas, Flammable Gas
ACGIH TLV: CL 0.3 ppm
DOT Classification: 2.3; Label: Poison Gas, Flammable Gas


