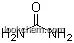Products Categories
| CAS No.: | 57-53-4 |
|---|---|
| Name: | Meprobamate |
| Article Data: | 22 |
| Molecular Structure: | |
|
|
|
| Formula: | C9H18 N2 O4 |
| Molecular Weight: | 218.253 |
| Synonyms: | 1,3-Propanediol,2-methyl-2-propyl-, dicarbamate (8CI,9CI); 2,2-Di(carbamoyloxymethyl)pentane;2-Methyl-2-n-propyl-1,3-propanediol dicarbamate;2-Methyl-2-propyl-1,3-propanediol dicarbamate; Amepromat; Amosene; Anastress;Anatimon; Andaxin; Aneural; Ansiatan; Anzil; Apascil; Appetrol; Arcoban;Artolon; Atraxin; Ayeramate; Bamo 400; Biobamat; Biobamate; Calmax; Calmiren;Canquil-400; Cap-O-Tran; Carb-A-Med; Carbamic acid2-methyl-2-propyltrimethylene ester; Carbaxin; Cirpon; Crestanil; Cyrpon;Dicandiol; Diron; Ecuanil; Epikur; Equanil; Equanil Suspension; Equinil;Fas-Cile 200; Gadexyl; Harmonin; Hartol; Holbamate; Kesso-Bamate; Klort;Larten; Lepetown; Libiolan; Mar-Bate; Mepantin; Mepavlon; Meposed; Mepr;Meprin; Meprindon; Meprobam; Meprobamat; Meprobamat-Petrasch; Meprobamate;Meproban; Meprocompren; Meprocon CMC; Meprol; Mepronil; Meprosin; Meprospan;Meprotabs; Meprotan; Meproten; Meprotil; Meptran; Mesmar; Metranquil;Micrainin; Microbamat; Milprem; Miltaun; Miltown; Miltrate; Morbam; My-trans;NSC 30418; Nervonus; Oasil; PMB 200; PMB 400; Panediol; Perequil; Perquietil;Pertranquil; Placidon; Placitate; Probamyl; Procalmadiol; Procalmidol; Promate;Protran; Quaname; Quanil; Rastenil; Reostral; Restenil; Robamate; Sedanil;Sedazil; Seril; Setran; Sowell; Tamate; Trankvilan; Tranlisant; Tranquilan;Tranquilax; Tranquiline; Urbil; Urbilat; Vio-Bamate |
| EINECS: | 200-337-5 |
| Density: | 1.139g/cm3 |
| Melting Point: | 104 - 106 C |
| Boiling Point: | 434.2°Cat760mmHg |
| Flash Point: | 229.7°C |
| Solubility: | negligible |
| Appearance: | white crystalline powder |
| Hazard Symbols: |

|
| Risk Codes: | R22 |
| Safety: | Human poison by unspecified routes. Moderately toxic to humans and experimentally by ingestion. Experimental poison by intravenous, intraperitoneal, and subcutaneous routes. An experimental teratogen. Human systemic effects by ingestion: coma, blood pressure decrease, regional or general arteriolar constriction, dyspnea, cyanosis, respiratory depression, nausea or vomiting, and allergic skin dermatitis. Experimental reproductive effects. Mutation data reported. Implicated in aplastic anemia. Used as a tranquilizer. When heated to decomposition it emits toxic fumes of NOx. See also CARBAMATES. |
| PSA: | 104.64000 |
| LogP: | 2.38400 |
- 81281-59-67-Benzylideneaminotheophylline
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 73441-42-6METHYL-5-CHLORO-2,2-DIMETHYLVALERATE
- 68439-39-4Poly(oxy-1,2-ethanediyl), alpha-(2-ethylhexyl)-omega-hydroxy-,
- 852475-26-4MC1568
- 958254-66-51H-Imidazo[4,5-b]pyridine-2-carboxaldehyde, 1-methyl-, hydrochloride
- 99170-93-1N-Methyl-2-oxazolamine
- 717878-06-31-(4-fluorophenyl)-4-nitro-1H-imidazole
- 914458-26-7[5-(2-fluorophenyl)-1-pentyl-1H-pyrrol-3-yl]-1-naphthalenyl-Methanone
- 894852-01-87-BROMO-2,2-DIMETHYL-2H-PYRIDO[3,2-B][1,4]OXAZIN-3(4H)-ONE
 This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
Chemistry
Empirical Formula: C9H18N2O4
Molecular Weight: 218.2502
EINECS: 200-337-5
Index of Refraction: 1.479
Density: 1.139 g/cm3
Flash Point: 229.7 °C
Storage temp: 2-8°C
Enthalpy of Vaporization: 69.03 kJ/mol
Boiling Point: 434.2 °C at 760 mmHg
Vapour Pressure: 9.64E-08 mmHg at 25 °C
Structure of Meprobamate (CAS NO.57-53-4):

History
Meprobamate (CAS NO.57-53-4) was first synthesized by Bernard John Ludwig, PhD, and Frank Milan Berger, MD, at Carter Products in May 1950. And, it rapidly became the first blockbuster psychotropic drug in American history, gaining notoriety for its seemingly miraculous effects and becoming popular in Hollywood. In the mid-1940s, Dr. Berger was working in a laboratory of a British drug company for looking for a preservative for penicillin. After moving to Wallace Laboratories in New Jersey, Dr. Berger and a chemist, Dr. Bernard Ludwig, synthesized a chemically-related tranquilizing compound, meprobamate, that was able to overcome these three drawbacks. A December 1955 study of 101 patients at the Mississippi State Hospital in Whitfield, Mississippi, found meprobamate useful in the alleviation of "mental symptoms."In April 1965 meprobamate was removed from the list of tranquilizers when experts ruled that the drug was a sedative instead. The significance of meprobamate and the benzodiazepines lies in the fact that these drugs, despite being habit-forming, essentially replaced the widely-used and potentially-lethal class of sedatives.
Uses
Meprobamate (CAS NO.57-53-4) is used as an anxiolytic drug. It can be used as a weak antipsychotic for the treatment of neurosis nervous insomnia.
Production
Take 2 - Methyl-amyl aldehydes with Formaldehyde for the reaction of aldol condensation to get 2 - Methyl -2 - propyl propylene glycol , and then having the reaction withe Phosgene and Ammonia, or with Sodium cyanate and Hydrochloric acid to derive: Meprobamate (CAS NO.57-53-4).
Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| child | TDLo | oral | 80mg/kg (80mg/kg) | BEHAVIORAL: COMA | Human Toxicology. Vol. 4, Pg. 215, 1985. |
| guinea pig | LD50 | subcutaneous | 380mg/kg (380mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 137, Pg. 375, 1962. | |
| hamster | LD50 | intraperitoneal | 625mg/kg (625mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: MUSCLE WEAKNESS BEHAVIORAL: ATAXIA | Journal of Pharmacology and Experimental Therapeutics. Vol. 129, Pg. 75, 1960. |
| hamster | LD50 | oral | 1410mg/kg (1410mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: ATAXIA BEHAVIORAL: MUSCLE WEAKNESS | Journal of Pharmacology and Experimental Therapeutics. Vol. 129, Pg. 75, 1960. |
| human | TDLo | oral | 280mg/kg (280mg/kg) | BEHAVIORAL: COMA VASCULAR: BP LOWERING NOT CHARACTERIZED IN AUTONOMIC SECTION LUNGS, THORAX, OR RESPIRATION: CYANOSIS | Ohio State Medical Journal. Vol. 52, Pg. 1304, 1956. |
| man | LDLo | unreported | 441mg/kg (441mg/kg) | "Poisoning; Toxicology, Symptoms, Treatments," 2nd ed., Arena, J.M., Springfield, IL, C.C. Thomas, 1970Vol. 2, Pg. 73, 1970. | |
| man | TDLo | oral | 5700ug/kg (5.7mg/kg) | LUNGS, THORAX, OR RESPIRATION: DYSPNEA GASTROINTESTINAL: NAUSEA OR VOMITING SKIN AND APPENDAGES (SKIN): "DERMATITIS, ALLERGIC: AFTER SYSTEMIC EXPOSURE" | Journal of the Irish Medical Association. Vol. 41, Pg. 119, 1957. |
| man | TDLo | oral | 114mg/kg (114mg/kg) | BEHAVIORAL: COMA CARDIAC: ARRHYTHMIAS (INCLUDING CHANGES IN CONDUCTION) LUNGS, THORAX, OR RESPIRATION: ACUTE PULMONARY EDEMA | Clinical Toxicology. Vol. 11, Pg. 501, 1977. |
| mouse | LD50 | intraperitoneal | 331mg/kg (331mg/kg) | Dissertationes Pharmaceuticae et Pharmacologicae. Vol. 23, Pg. 281, 1971. | |
| mouse | LD50 | intravenous | 230mg/kg (230mg/kg) | European Journal of Medicinal Chemistry--Chimie Therapeutique. Vol. 12, Pg. 447, 1977. | |
| mouse | LD50 | oral | 750mg/kg (750mg/kg) | Journal of Medicinal Chemistry. Vol. 15, Pg. 998, 1972. | |
| mouse | LD50 | subcutaneous | 520mg/kg (520mg/kg) | Journal of Pharmacy and Pharmacology. Vol. 26, Pg. 109, 1974. | |
| rabbit | LD50 | intravenous | 260mg/kg (260mg/kg) | SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: REGIDITY | International Journal of Neuropharmacology. Vol. 5, Pg. 305, 1966. |
| rat | LD50 | intraperitoneal | 410mg/kg (410mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: MUSCLE WEAKNESS BEHAVIORAL: ATAXIA | Journal of Pharmacology and Experimental Therapeutics. Vol. 129, Pg. 75, 1960. |
| rat | LD50 | intravenous | 350mg/kg (350mg/kg) | BEHAVIORAL: MUSCLE WEAKNESS LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Prensa Medica Argentina. Vol. 44, Pg. 915, 1957. |
| rat | LD50 | oral | 794mg/kg (794mg/kg) | Toxicology and Applied Pharmacology. Vol. 19, Pg. 93, 1971. | |
| rat | LD50 | subcutaneous | 525mg/kg (525mg/kg) | Nippon Yakurigaku Zasshi. Japanese Journal of Pharmacology. Vol. 56, Pg. 377, 1960. | |
| women | LDLo | oral | 760mg/kg (760mg/kg) | BEHAVIORAL: COMA | JAMA, Journal of the American Medical Association. Vol. 207, Pg. 361, 1969. |
| women | TDLo | oral | 112mg/kg (112mg/kg) | BEHAVIORAL: ATAXIA BEHAVIORAL: COMA | Clinical Toxicology. Vol. 11, Pg. 501, 1977. |
| women | TDLo | oral | 120mg/kg (120mg/kg) | BEHAVIORAL: COMA CARDIAC: EKG CHANGES NOT DIAGNOSTIC OF ABOVE | Clinical Toxicology. Vol. 11, Pg. 501, 1977. |
| women | TDLo | oral | 240mg/kg (240mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: COMA VASCULAR: BP ELEVATION NOT CHARACTERIZED IN AUTONOMIC SECTION | Clinical Toxicology. Vol. 11, Pg. 501, 1977. |
| women | TDLo | oral | 360mg/kg (360mg/kg) | BEHAVIORAL: COMA LUNGS, THORAX, OR RESPIRATION: RESPIRATORY STIMULATION LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Clinical Toxicology. Vol. 11, Pg. 501, 1977. |
| women | TDLo | oral | 384mg/kg (384mg/kg) | VASCULAR: BP LOWERING NOT CHARACTERIZED IN AUTONOMIC SECTION VASCULAR: REGIONAL OR GENERAL ARTERIOLAR CONSTRICTION LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Northwest Medicine. Vol. 56, Pg. 321, 1957. |
Consensus Reports
Reported in EPA TSCA Inventory. EPA Genetic Toxicology Program.
Safety Profile
Human poison by unspecified routes. Moderately toxic to humans and experimentally by ingestion. Experimental poison by intravenous, intraperitoneal, and subcutaneous routes. An experimental teratogen. Human systemic effects by ingestion: coma, blood pressure decrease, regional or general arteriolar constriction, dyspnea, cyanosis, respiratory depression, nausea or vomiting, and allergic skin dermatitis. Experimental reproductive effects. Mutation data reported. Implicated in aplastic anemia. Used as a tranquilizer. When heated to decomposition it emits toxic fumes of NOx. See also CARBAMATES.
Hazard Codes:  Xn
Xn  T
T F
F
Risk Statements: 22-39/23/24/25-23/24/25-11
R11:Highly flammable.
R22:Harmful if swallowed.
R23/24/25:Toxic by inhalation, in contact with skin and if swallowed.
R39:Danger of very serious irreversible effects.
Safety Statements: 7-16-36/37-45
S7:Keep container tightly closed.
S16:Keep away from sources of ignition.
S36/37:Wear suitable protective clothing and gloves.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
Specification
Meprobamate ,its cas register number is 57-53-4. It also can be called Miltown ; Equanil ; Meprospan ; Tranmep and 1,3-Propanediol, 2-methyl-2-propyl-, dicarbamate . It was the best-selling minor tranquilizer for a time, but has largely been replaced by the benzodiazepines. It is a carbamate derivative. Although it was the best-selling minor tranquilizer for a time, it has largely been replaced by the benzodiazepines. Meprobamate has most of the pharmacological effects and dangers of the barbiturates and is licensed for the short-term relief of anxiety, although it is not known whether the purported anti-anxiety effects of meprobamate are separable from its sedative effects.