Products Categories
| CAS No.: | 7790-91-2 |
|---|---|
| Name: | CHLORINE TRIFLUORIDE |
| Molecular Structure: | |
|
|
|
| Formula: | ClF3 |
| Molecular Weight: | 92.4482 |
| Synonyms: | Chlorinefluoride (Cl2F6); Chlorine trifluoride; Chlorine trifluoride (ClF3);Chlorotrifluoride; Trifluorochlorine |
| EINECS: | 232-230-4 |
| Density: | 3.18 (Relative vapour density (air = 1)) |
| Melting Point: | -76ºC |
| Boiling Point: | 12ºC |
| Solubility: | reaction in water |
| Appearance: | NEARLY COLOURLESS COMPRESSED LIQUEFIED GAS , WITH CHARACTERISTIC ODOUR. |
| Hazard Symbols: | Explodes in contact with organic materials or with water. Dangerous fire risk. A poison, very toxic, corrosive to skin. TLV: ceiling 0.1 ppm. |
| Risk Codes: | 8-35 |
| Safety: |
Human poison by inhalation. An eye irritant. See also FLUORIDES, CHLORINE, and FLUORINE. Spontaneously flammable. A powerful oxidant which may react violently with oxidizable materials. A rocket propellant. Explosive reaction with water, bis(trifluoro methyl)sulfide or -disulfide, polychlorotrifluoroethylene, trifluoromethanesulfenyl chloride, and other hydrogen- containing materials (e.g., ammonia, coal gas, hydrogen, hydrogen sulfide, methane, acetic acid, benzene, ether, cotton, paper, wood). Forms shock-sensitive explosive mixtures with highly chlorinated compounds (e.g., carbon tetrachloride), nitroaryl compounds (e.g., trinitrotoluene, hexanitrobiphenyl, hexanitrodiphenyl amine, hexanitro diphenyl sulfide, hexanitrodiphenyl ether). Reaction with ammonium fluoride or ammonium hydrogen fluoride forms explosive gaseous products. Ignition on contact with boron-containing materials, iodine, finely divided refractory materials (e.g., asbestos, glass wool, sand, tungsten carbide), fluorinated polymers (with flowing trifluoride). Violent reaction with acids (e.g., nitric or sulfuric), chromium trioxide, ruthenium, selenium tetrafluoride (above 106°C), metals, metal oxides, metal salts, nonmetals, nonmetal salts, organic matter, glass wool, acetic acid, Al, Sb, As, Cu, Ir, Fe, Pb, Mg, Mo, Os, P, K, Rh, Se, Si, Ag, Na, S, Te, Sn, W, Zn, oxides, CO, graphite, HgI2, HNO3, K2CO3, KI, rubber, AgN3, AgNO3, NaOH, V2P5, WO3. Incompatible with fuels, nitro compounds. When heated to decomposition or in reaction with water or steam it emits toxic fumes of F− and Cl−. |
| PSA: | 0.00000 |
| LogP: | 1.95010 |
- 81281-59-67-Benzylideneaminotheophylline
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 852475-26-4MC1568
- 958254-66-51H-Imidazo[4,5-b]pyridine-2-carboxaldehyde, 1-methyl-, hydrochloride
- 99170-93-1N-Methyl-2-oxazolamine
- 914458-26-7[5-(2-fluorophenyl)-1-pentyl-1H-pyrrol-3-yl]-1-naphthalenyl-Methanone
- 894852-01-87-BROMO-2,2-DIMETHYL-2H-PYRIDO[3,2-B][1,4]OXAZIN-3(4H)-ONE
- 90221-55-92-bromo-5-methylbenzaldehyde
- 885590-99-82,3-DIFLUORO-4-IODOBENZALDEHYDE
- 97730-31-9(S)-4'-(2-Methylbutyl)Biphenyl-4-Carbonitrile

| Conditions | Yield |
|---|---|
| In gas at 119 - 232°C in Al-tubes about 30 - 45 min;; | A 95% B n/a |
| In gas at 170°C in Ni-tubes about 40 min;; | A 12% B n/a |
| nickel(II) fluoride In gas Kinetics; at 467, 497 and 530°K in Ni-tubes;; |


| Conditions | Yield |
|---|---|
| In neat (no solvent) equilibrium react.;; | |
| In neat (no solvent) Kinetics; equilibrium at 500 - 800°K;; | |
| In neat (no solvent) Kinetics; equilibrium at 100 - 380°C;; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) heated (nickel labyrinth crucible, dry N2 atm., heating rate 4-6 K/min.); |

| Conditions | Yield |
|---|---|
| In neat (no solvent) heated (nickel labyrinth crucible, dry N2 atm., heating rate 4-6 K/min.); |

| Conditions | Yield |
|---|---|
| In neat (no solvent) heating S2Cl2 and NaF under refluxat 136°C; hydrolysis at -120°C;; | 20-45 |

- 184851-42-1, 478167-78-1, 13637-63-3, 676353-68-7, 478167-76-9
chlorine pentafluoride

A
- 7790-91-2
chlorine trifluoride

B
- 7782-41-4
fluorine

| Conditions | Yield |
|---|---|
| In neat (no solvent) equilibrium;; | |
| In neat (no solvent) equilibrium between 211 - 271°K;; | |
| In neat (no solvent) equilibrium between 211 - 271°K;; | |
| In neat (no solvent) equilibrium;; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) dry air atmosphere; heating (90-150°C, 4-6 K/min); TGA; |

| Conditions | Yield |
|---|---|
| With sodium fluoride In melt placing the fluorides in a Monel cylinder with a Ni-dip tube and outlet for gaseous phase (3 cooper traps, temp. from dry ice to room temp.); protecting from atmosphere; heating with electric furnace to 725°C; streaming Cl2 through melt for 5h; detecting ClF along with ClF3 by IR spectroscopy; |

- 13773-81-4
krypton difluoride

A
- 7790-91-2
chlorine trifluoride

- 184851-42-1, 478167-78-1, 13637-63-3, 676353-68-7, 478167-76-9
chlorine pentafluoride

| Conditions | Yield |
|---|---|
| With polyethylene observed by IR and raman spectroscopy of KrF2;; |


| Conditions | Yield |
|---|---|
| other Radiation; α-radiation; | |
| other Radiation; α-radiation; |
- Total:1 Page 1 of 1 1

What can I do for you?
Get Best Price
Chemistry
Molecular Structure of Chlorine trifluoride (CAS NO. 7790-91-2):
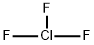
EINECS: 232-230-4
Chemical Name: Chlorine trifluoride
Molecular Formula: ClF3
XLogP3-AA: 2.5
H-Bond Donor: 0
H-Bond Acceptor: 3
InChI: InChI=1S/ClF3/c2-1(3)4
InChIKey: JOHWNGGYGAVMGU-UHFFFAOYSA-N
Density: 1.8 g/cm3
Molecular Weight: 92.448210 g/mol
Enthalpy of Vaporization: 27.53 kJ/mol
Boiling Point: 11.3 °C at 760 mmHg
Vapour Pressure: 1310 mmHg at 25 °C
Melting Point: -83 °C
Water Solubility: 7840 mg/L at 25 °C
Uses
Chlorine trifluoride is mainly used to produce uranium hexafluoride :
U + 3ClF3 → UF6 + 3ClF
Before the start of World War II, Chlorine trifluoride under the code name of N-stoff was investigated for military applications by the Kaiser Wilhelm Institute in Nazi German. It was found to be an effective combined incendiary weapon and poison gas. Now, it is never used in war. Chlorine trifluoride has also been investigated as a high-performance storable oxidizer in rocket propellant systems due to its hypergolic properties with every known fuel even things like cloth, wood, and test engineers. Chlorine trifluoride is used in the semiconductor industry to clean chemical vapour deposition chambers without having to dismantle the chamber. It does not need to be activated by plasma as the heat of the chamber is enough to make it decompose and react with the semiconductor material.
Production
Chlorine trifluoride was prepared by fluorination of chlorine first reported by Ruff and Krug. The reaction also produces ClF, then the mixture was separated by distillation.
3F2 + Cl2 → 2ClF3
Toxicity Data With Reference
| 1. | eye-rat 21 ppm/12H-I | AMIHAB AMA Archives of Industrial Health. 12 (1955),515. | ||
| 2. | eye-dog 21 ppm/12H-I | AMIHAB AMA Archives of Industrial Health. 12 (1955),515. | ||
| 3. | ihl-hmn LCLo:50 ppm | 34ZIAG Toxicology of Drugs and Chemicals, Deichmann, W.B.,New York, NY.: Academic Press, Inc.,1966,651. | ||
| 4. | ihl-rat LCLo:400 ppm/30M | TXAPA9 Toxicology and Applied Pharmacology. 27 (1974),527. | ||
| 5. | ihl-mus LC50:178 ppm/1H | AMRL** Aerospace Medical Research Laboratory Report. (Aerospace Technical Div., Air Force Systems Command, Wright-Patterson Air Force Base, OH 45433) TR-70-55 ,1970. | ||
| 6. | ibl-mky LC50:230 ppm/1H | AMRL** Aerospace Medical Research Laboratory Report. (Aerospace Technical Div., Air Force Systems Command, Wright-Patterson Air Force Base, OH 45433) TR-70-77 ,1970. |
Consensus Reports
Reported in EPA TSCA Inventory.
Safety Profile
Safety Information of Chlorine trifluoride (CAS NO. 7790-91-2):
Hazard Codes: O 
Hazard Note: Oxidising agent
Risk Statements: 8-35
R8 :Contact with combustible material may cause fire
R35:Causes severe burns
Safety Statements: 17-38
S17:Keep away from combustible material
S38:In case of insufficient ventilation, wear suitable respiratory equipment
RIDADR: 1749
HazardClass: 2.3
Human poison by inhalation. An eye irritant. See also FLUORIDES, CHLORINE, and FLUORINE. Spontaneously flammable. A powerful oxidant which may react violently with oxidizable materials. A rocket propellant.
Explosive reaction with water, bis(trifluoro methyl)sulfide or -disulfide, polychlorotrifluoroethylene, trifluoromethanesulfenyl chloride, and other hydrogen- containing materials (e.g., ammonia, coal gas, hydrogen, hydrogen sulfide, methane, acetic acid, benzene, ether, cotton, paper, wood). Forms shock-sensitive explosive mixtures with highly chlorinated compounds (e.g., carbon tetrachloride), nitroaryl compounds (e.g., trinitrotoluene, hexanitrobiphenyl, hexanitrodiphenyl amine, hexanitro diphenyl sulfide, hexanitrodiphenyl ether). Reaction with ammonium fluoride or ammonium hydrogen fluoride forms explosive gaseous products.
Ignition on contact with boron-containing materials, iodine, finely divided refractory materials (e.g., asbestos, glass wool, sand, tungsten carbide), fluorinated polymers (with flowing trifluoride).
Violent reaction with acids (e.g., nitric or sulfuric), chromium trioxide, ruthenium, selenium tetrafluoride (above 106°C), metals, metal oxides, metal salts, nonmetals, nonmetal salts, organic matter, glass wool, acetic acid, Al, Sb, As, Cu, Ir, Fe, Pb, Mg, Mo, Os, P, K, Rh, Se, Si, Ag, Na, S, Te, Sn, W, Zn, oxides, CO, graphite, HgI2, HNO3, K2CO3, KI, rubber, AgN3, AgNO3, NaOH, V2P5, WO3. Incompatible with fuels, nitro compounds. When heated to decomposition or in reaction with water or steam it emits toxic fumes of F− and Cl−.
Standards and Recommendations
OSHA PEL: CL 0.1 ppm
ACGIH TLV: CL 0.1 ppm
DFG MAK: 0.1 ppm (0.38 mg/m3)
DOT Classification: 2.3; Label: Poison Gas, Oxidizer, Corrosive
Specification
Chlorine trifluoride with cas registry number of 7790-91-2 is also called for Chlorine fluoride ; Chlorine fluoride (ClF3) ; Chlorine trifluoride (ClF3) ; Chlorotrifluoride ; HSDB 970 ; Trifluorure de chlore ; Trifluorure de chlore [French] ; Chlorine trifluoride [UN1749] [Poison gas] ; UN1749 . It is A colorless gas or green liquid with a pungent odor. Pure Chlorine trifluoride is stable to 180° in glass vessels; above this temperature it decomposes by a free radical mechanism to the elements.
