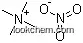1941-24-8Relevant articles and documents
Tetraalkylammonium Salts of Platinum Nitrato Complexes: Isolation, Structure, and Relevance to the Preparation of PtOx/CeO2 Catalysts for Lowerature CO Oxidation
Vasilchenko, Danila,Topchiyan, Polina,Berdyugin, Semen,Filatov, Evgeny,Tkachev, Sergey,Baidina, Iraida,Komarov, Vladislav,Slavinskaya, Elena,Stadnichenko, Andrey,Gerasimov, Evgeny
, p. 6075 - 6087 (2019)
A series of tetraalkylammonium salts with anionic platinum nitrato complexes (Me4N)2[Pt2(μ-OH)2(NO3)8] (1), (Et4N)2[Pt2(μ-OH)2(NO3)8] (2), (n-Pr4N)2[Pt2(μ-OH)2(NO3)8] (3b), (n-Pr4N)2[Pt(NO3)6] (3a), and (n-Bu4N)2[Pt(NO3)6] (4) were isolated from nitric acid solutions of [Pt(H2O)2(OH)4] in high yield. The structures of salts 2, 3a, 3b, and 4, prepared for the first time, were characterized by X-ray diffraction. The sorption of [Pt(NO3)6]2- and [Pt2(μ-OH)2(NO3)8]2- complexes onto the ceria surface from acetone solutions of salts 4 and 1 was examined. The dimeric anion was shown to quickly and irreversibly chemisorb onto the CeO2 carrier, selectively transforming into Pt(II) centers after thermal treatment, becoming active in the lowerature CO oxidation reaction (T50% = 110 °C at a space velocity of 240 000 h-1). By contrast, the homoleptic complex [Pt(NO3)6]2- did not interact with the ceria, which may be attributed to the substitutional inertness of the [Pt(NO3)6]2- anion. We believe that the strategy based on the sorption of polynuclear platinum nitrato complexes is an effective route to prepare ionic platinum species uniformly distributed on an oxide carrier for various catalytic applications.
A calorimetric study of the hydrolysis and peroxide complex formation of the uranyl(vi) ion
Zanonato, Pier Luigi,Di Bernardo, Plinio,Grenthe, Ingmar
, p. 2378 - 2383 (2014/02/14)
The enthalpies of reaction for the formation of uranyl(vi) hydroxide {[(UO2)2(OH)2]2+, [(UO 2)3(OH)4]2+, [(UO2) 3(OH)5]+, [(UO2)3(OH) 6](aq), [(UO2)3(OH) 7]-, [(UO2)3(OH)8] 2-, [(UO2)(OH)3]-, [(UO 2)(OH)4]2-} and peroxide complexes {[UO 2(O2)(OH)]- and [(UO2) 2(O2)2(OH)]-} have been determined from calorimetric titrations at 25 °C in a 0.100 M tetramethyl ammonium nitrate ionic medium. The hydroxide data have been used to test the consistency of the extensive thermodynamic database published by the Nuclear Energy Agency (I. Grenthe, J. Fuger, R. J. M. Konings, R. J. Lemire, A. B. Mueller, C. Nguyen-Trung and H. Wanner, Chemical Thermodynamics of Uranium, North-Holland, Amsterdam, 1992 and R. Guillaumont, T. Fanghaenel, J. Fuger, I. Grenthe, V. Neck, D. J. Palmer and M. R. Rand, Update on the Chemical Thermodynamics of Uranium, Neptunium, Plutonium, Americium and Technetium, Elsevier, Amsterdam, 2003). A brief discussion is given about a possible structural relationship between the trinuclear complexes [(UO2)3(OH) n]6-n, n = 4-8.
The synthesis of quaternary ammonium salts from ammonium salts and dialkyl carbonate
Zheng, Zhuoqun,Wu, Tinghua,Zhou, Xiaoping
, p. 1864 - 1865 (2008/03/14)
Quaternary ammonium salts were synthesized from ammonium salts and dialkyl carbonates over an ionic liquid catalyst 1-ethyl-3-methylimidazolium bromide. The Royal Society of Chemistry 2006.