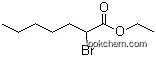
5333-88-0Relevant articles and documents
Efficient and Divergent Synthesis of α-Halogenated Amides and Esters by Double Electrophilic Activation of Ynamides
Thilmany, Pierre,Evano, Gwilherm
supporting information, p. 242 - 246 (2019/11/26)
An efficient and modular entry to α-halogenated amides and esters is reported. This reaction is based on an underestimated double electrophilic activation of ynamides sequentially involving highly reactive activated keteniminium and iminium ions. Upon simple reaction with HCl and an electrophilic halogenation reagent in the presence of water or an alcohol, a broad range of ynamides can be transformed, in a highly divergent manner, to α-halo amides and esters with high efficiency and under mild conditions.
Enantiodiscrimination of racemic electrophiles by diketopiperazine enolates: asymmetric synthesis of methyl 2-amino-3-aryl-butanoates and 3-methyl-aspartates
Bull, Steven D.,Davies, Stephen G.,Epstein, Simon W.,Garner, A. Christopher,Mujtaba, Nadeam,Roberts, Paul M.,Savory, Edward D.,Smith, Andrew D.,Tamayo, Juan A.,Watkin, David J.
, p. 7911 - 7925 (2007/10/03)
Enolates of (S)-N,N′-bis-(p-methoxybenzyl)-3-iso-propylpiperazine-2,5-dione exhibit high levels of enantiodiscrimination in alkylations with (RS)-1-aryl-1-bromoethanes and (RS)-2-bromoesters, affording substituted diketopiperazines containing two new stereogenic centres in high de. Deprotection and hydrolysis of the resultant substituted diketopiperazines provides a route to the asymmetric synthesis of homochiral methyl 2-amino-3-aryl-butanoates and 3-methyl-aspartates in high de and ee.
Oxidation of Diols and Ethers by NaBrO3/NaHSO3 Reagent
Sakaguchi, Satoshi,Kikuchi, Daisuke,Ishii, Yasutaka
, p. 2561 - 2566 (2007/10/03)
NaBrO3 combined with NaHSO3 was found to be an excellent oxidizing reagent of alcohols, diols, and ethers under mild conditions. A variety of aliphatic and cyclic diols were selectively oxidized with satisfactory yields to the corresponding hydroxy ketones and/or diketones, which are difficult to selectively prepare due to a concomitant formation of cleaved products. For example, 2-hydroxycyclohexanone and 1,2-cyclohexanedione were selectively formed by allowing 1,2-cyclohexanediol to react with NaBrO3/NaHSO3 reagent in a selected solvent. On the other hand, an alkyl ether, such as dioctyl ether, reacted with NaBrO3/NaHSO3, in water at room temperature to give octyl octanoate in 82% yield. The same oxidation at higher temperature (60°C) produced the α-brominated ester, octyl 2-bromooctanoate, which is considered to be formed through an alkenyl alkyl ether as the intermediate. The treatment of 1-ethoxy-l-heptene with NaBrO3/NaHSO3 afforded ethyl 2-bromoheptanoate and 2-bromoheptanoic acid as the major products.