63610-09-3 Usage
Description
Lodoxamide tromethamine was launched as an ophthalmic formulation for the
treatment of allergic conjunctivitis. It appears to act as a mast cell stabilizer inhibiting
degranulation and the release of histamine and other vasoactive products in a similar
manner to sodium cromoglycate. The compound is also under investigation as an
orally active antiallergic/antiasthmatic agent.
Originator
Upjohn (U.S.A.)
Uses
cataract treatment
Brand name
Alomide (Alcon);Almide.
General Description
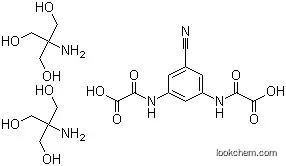
Lodoxamidetromethamine, N, N -(2-chloro-5-cyano-m-phenylene)dioxamicacid (Alomide), is a white crystalline, water-solublepowder. The only significant structural similarity betweenlodoxamide and cromolyn and nedocromil is the presence oftwo acidic groups. Lodoxamide is indicated in the treatmentof the ocular disorders including vernal keratoconjunctivitis,vernal conjunctivitis, and vernal keratitis. Lodoxamide isavailable as a 0.1% solution, with each milliliter containing1.78 mg of lodoxamide tromethamine equivalent to 1 mg oflodoxamide. The solution contains the preservative benzalkoniumchloride (0.007%) as well as mannitol, hydroxypropylmethylcellulose, sodium citrate, citric acid, edetatedisodium, tyloxapol, hydrochloric acid and/or sodium hydroxide(to adjust pH), and purified water.The dose for adults and children older than 2 years of ageis 1 to 2 drops in each affected eye 4 times daily for up to 3months. The most frequently reported ocular adverse experienceswere transient burning, stinging, or discomfort oninstillation.
Check Digit Verification of cas no
The CAS Registry Mumber 63610-09-3 includes 8 digits separated into 3 groups by hyphens. The first part of the number,starting from the left, has 5 digits, 6,3,6,1 and 0 respectively; the second part has 2 digits, 0 and 9 respectively.
Calculate Digit Verification of CAS Registry Number 63610-09:
(7*6)+(6*3)+(5*6)+(4*1)+(3*0)+(2*0)+(1*9)=103
103 % 10 = 3
So 63610-09-3 is a valid CAS Registry Number.
InChI:InChI=1/C11H6ClN3O6.C4H11NO3/c12-7-5(14-8(16)10(18)19)1-4(3-13)2-6(7)15-9(17)11(20)21;5-4(1-6,2-7)3-8/h1-2H,(H,14,16)(H,15,17)(H,18,19)(H,20,21);6-8H,1-3,5H2
63610-09-3Relevant articles and documents
Preparation method of lodoxamide tromethamine intermediate
-
Paragraph 0048; 0049, (2018/12/13)
The invention belongs to the technical field of medicine chemical engineering, and particularly discloses a preparation method of a lodoxamide tromethamine intermediate of lodoxamide. The method comprises the following steps of (1) dissolving 3,5-diamino-4-chlorobenzonitrile into an organic solvent to form a solution A; (2) dissolving oxalyl chloride into the organic solvent to form a solution B;(3) slowly dripping the solution A into the solution B; in the dripping process, controlling the temperature of the reaction liquid at -20 to 0 DEG C; after the reaction is completed, slowly drippinga certain amount of ice water into the reaction liquid; after the dripping is completed, continuously performing stirring for 0.5 to 1h; then, performing suction filtering; drying obtained filter caketo obtain lodoxamide. The reaction steps of the method are few; the operation is facilitated; the yield is greatly improved and can reach 90 percent or higher; meanwhile, the related impurities of the product lodoxamide are few; the product purity is high; the HPLC purity can reach 99.5 percent or higher; great significance is realized on the synthesis of the lodoxamide tromethamine medicine.