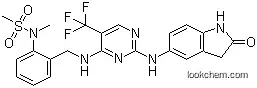
717906-29-1 Usage
Description
Proline-rich tyrosine kinase 2 (PYK2) and focal adhesion kinase (FAK) are nonreceptor tyrosine kinases that constitute the focal adhesion kinase subfamily. PYK2 is expressed in both bone-forming osteoblasts and bone-resorbing osteoclasts where it has a positive role in osteoclast maturation and bone resorption. PF-431396 is a pyrimidine-based dual inhibitor of FAK and PYK2 (IC50s = 2 and 11 nM, respectively). In ovariectomized rats, it has been shown to increase bone formation, promoting osteoblast recruitment and activity.
Uses
Different sources of media describe the Uses of 717906-29-1 differently. You can refer to the following data:
1. PF 431396 is a dual focal adhesion kinase (FAK) and proline-rich tyrosine kinase 2 (PYK2) inhibitor (IC50 values are 2 and 11 nM respectively). Promotes osteoblast recruitment and activity, and stimulates bone formation in ovariectomized rats.
2. PF-431396 hydrate has been used as a dual inhibitor of proline-rich tyrosine kinase 2 (PYK2) / focal adhesion kinase (FAK) inhibitor to study its effects on phosphorylation of Yes-associated protein (YAP) at Ser127 and steady state of transcriptional coactivator with PDZ-binding motif (TAZ) . It has also been used as a PYK2/FAK inhibitor to study its effects on protein kinase A activation in human sperm samples .
Biochem/physiol Actions
PF-431396 is a potent inhibitor of PYK2 and FAK kinases (IC50 = 11 and 1.5 nM, respectively). PF-431396 increases bone formation and protects against bone loss in ovariectomized rats.
Check Digit Verification of cas no
The CAS Registry Mumber 717906-29-1 includes 9 digits separated into 3 groups by hyphens. The first part of the number,starting from the left, has 6 digits, 7,1,7,9,0 and 6 respectively; the second part has 2 digits, 2 and 9 respectively.
Calculate Digit Verification of CAS Registry Number 717906-29:
(8*7)+(7*1)+(6*7)+(5*9)+(4*0)+(3*6)+(2*2)+(1*9)=181
181 % 10 = 1
So 717906-29-1 is a valid CAS Registry Number.
InChI:InChI=1S/C22H21F3N6O3S/c1-31(35(2,33)34)18-6-4-3-5-13(18)11-26-20-16(22(23,24)25)12-27-21(30-20)28-15-7-8-17-14(9-15)10-19(32)29-17/h3-9,12H,10-11H2,1-2H3,(H,29,32)(H2,26,27,28,30)
717906-29-1Relevant articles and documents
Trifluoromethylpyrimidine-based inhibitors of proline-rich tyrosine kinase 2 (PYK2): Structure-activity relationships and strategies for the elimination of reactive metabolite formation
Walker, Daniel P.,Christopher Bi,Kalgutkar, Amit S.,Bauman, Jonathan N.,Zhao, Sabrina X.,Soglia, John R.,Aspnes, Gary E.,Kung, Daniel W.,Klug-McLeod, Jacquelyn,Zawistoski, Michael P.,McGlynn, Molly A.,Oliver, Robert,Dunn, Matthew,Li, Jian-Cheng,Richter, Daniel T.,Cooper, Beth A.,Kath, John C.,Hulford, Catherine A.,Autry, Christopher L.,Luzzio, Michael J.,Ung, Ethan J.,Roberts, W. Gregory,Bonnette, Peter C.,Buckbinder, Leonard,Mistry, Anil,Griffor, Matthew C.,Han, Seungil,Guzman-Perez, Angel
scheme or table, p. 6071 - 6077 (2009/08/07)
The synthesis and SAR for a series of diaminopyrimidines as PYK2 inhibitors are described. Using a combination of library and traditional medicinal chemistry techniques, a FAK-selective chemical series was transformed into compounds possessing good PYK2 potency and 10- to 20-fold selectivity against FAK. Subsequent studies found that the majority of the compounds were positive in a reactive metabolite assay, an indicator for potential toxicological liabilities. Based on the proposed mechanism for bioactivation, as well as a combination of structure-based drug design and traditional medicinal chemistry techniques, a follow-up series of PYK2 inhibitors was identified that maintained PYK2 potency, FAK selectivity and HLM stability, yet were negative in the RM assay.