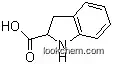
78348-24-0Relevant articles and documents
Semi-rational protein engineering of a novel esterase from Bacillus aryabhattai (BaCE) for resolution of (R,S)-ethyl indoline-2-carboxylate to prepare (S)-indoline-2-carboxylic acid
Zhang, Hongjun,Cheng, Zeguang,Wei, Litian,Yu, Xinjun,Wang, Zhao,Zhang, Yinjun
, (2022/01/24)
A gene encoding an esterase from Bacillus aryabhattai (BaCE) was identified, synthesized and efficiently expressed in the Escherichia coli system. A semi-rational protein engineering was applied to further improve the enzyme's enantioselectivity. Under the guidance of the molecular docking result, a single mutant BaCE-L86Q and a double mutant BaCE-L86Q/G284E were obtained, with its Emax value 6.4 times and 13.9 times of the wild-type BaCE, respectively. The recombinant BaCEs were purified and characterized. The overwhelming E value demonstrated that BaCE-L86Q/G284E was a promising biocatalyst for the biological resolution to prepare (S)-indoline-2-carboxylic acid.
Of enantiomerically enriched indoline - 2 - formic acid
-
, (2017/09/01)
The invention discloses a synthesis method of enantiomer-enriched indoline-2-formic acid shown in a formula (I). The synthesis method of the enantiomer-enriched indoline-2-formic acid comprises the following steps: by adopting low-cost and available ortho-position halogen substituted benzaldehyde and N-benzoyl substituted glycine as starting materials, carrying out Erlenmeyer-Plochl cyclization, alkaline hydrolysis and asymmetric catalytic hydrogen for constructing a chiral center, and then carrying out acid catalysis, deprotection and cyclization sequentially or cyclization, acid catalysis and deprotection sequentially, so that the enantiomer-enriched indoline-2-formic acid is obtained. The synthesis method of the enantiomer-enriched indoline-2-formic acid has the advantages that raw materials used in the whole process route are low-cost and easily available, harmful substances or multiple danger special processes are not used, reaction conditions are mild, technological operation is simple, production is safe and stable, the product yield is high, the purity is high, less three wastes are produced, and the energy consumption is low, so that the synthesis method of the enantiomer-enriched indoline-2-formic acid is a process route especially applicable to industrial production. The formula (1) is described in the specification.
Preparation method and application of hydrazide indoles drugs
-
Paragraph 0037; 0038; 0041, (2017/08/28)
The invention provides a preparation method and application of hydrazide indoles drugs, and discloses a 2-hydrazide substituted indole compound and a preparation method and application thereof. The novel 2-hydrazide substituted indole compound has quite high activity of inhibiting growth of tumor cells, and particularly has remarkable inhibiting effect on growth of human rectal cancer cells and colon cancer cells which have vascular endothelial growth factor receptor-2 subtype high expression; the IC50 value of the novel 2-hydrazide substituted indole compound can be about 10 mu M; the novel 2-hydrazide substituted indole compound has good antiangiogensis activity on a CAM (chick chorioallantoic membrane) model; and moreover, the compound 21 has good capability of resisting cell proliferation after HUVEC is stimulated by VEGF and specificity of resisting proliferation after HUVEC is simulated by VEGF.