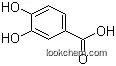
99-50-3 Usage
Description
Protocatechuic acid (3,4-dihydroxybenzoic acid, PCA) is a simple phenolic acid. It is found in a large variety of edible plants and possesses various pharmacological activities.Protocatechuic acid has an antibacterial effect, and has different degrees of antibacterial effect on Pseudomonas aeruginosa, Escherichia coli, Typhoid bacillus, Shigella, Alcaligenes, Bacillus subtilis and Staphylococcus aureus in vitro. It also has expectorant and anti-asthmatic effects. It is clinically used to treat chronic bronchitis.
Physical properties
Protocatechuic acid has a density of 1.54g/cm3 and a melting point of about 200°C. It is soluble in ethanol, ether, acetone, ethyl acetate, soluble in hot water, slightly soluble in cold water, and insoluble in benzene and chloroform. Decomposes in boiling water and emits carbon dioxide. Its aqueous solution is green when it meets ferric chloride, and red when it meets sodium bicarbonate. It is a white to brown crystalline powder that will change color in the air.
Pharmaceutical Applications
Protocatechuic acid is a metabolite of complex polyphenols, such as anthocyanins and proanthocyanidins. They are high in plants and fruits and can be absorbed by animals and humans. It is estimated that people’s daily intake of anthocyanins is higher than other anthocyanins. Polyphenols are higher, so the nutritional value of protocatechuic acid is increasingly recognized. A large number of experiments have proved that protocatechuic acid has a variety of biological activities against different molecular targets. It has antibacterial, antioxidant, anti-inflammatory, anti-hyperglycemic and neuroprotective effects. In addition, protocatechin has potential chemical Protective effect, it can inhibit chemical carcinogens in vitro and produce pro-apoptosis and anti-proliferation effects in different aspects.
Preparation
1.Preparation of piperonic acidAdd 12 g piperonal and 300 ml water into a 1000 ml three-necked flask, heat it to 70~80℃, and under vigorous stirring, add 5% potassium permanganate aqueous solution (18 g potassium permanganate plus 360 ml Water), the dripping time is about 30 minutes, after the addition, continue to stir until the purple fades, filter while hot, and wash the filter cake. The mother liquor is acidified and filtered to obtain piperonic acid. The finished product is naturally dried 12.4 g, the yield is 94%, and the melting point is 228~231℃.2. Preparation of protocatechuic acidAdd 4 g of aluminum trichloride to 150 ml of nitrobenzene, stir to dissolve, add 10 g of piperonic acid in batches, stir at room temperature for 3 to 5 h, pour the reaction solution into 100 ml of water, separate the water layer, Sulfur dioxide was introduced into the aqueous solution, and protocatechuic acid was precipitated. The dry weight is 7.5g, the melting point is 200~201℃, and the yield is 81%.
Chemical Properties
white to light yellow crystal powder
Uses
Different sources of media describe the Uses of 99-50-3 differently. You can refer to the following data:
1. 3,4-Dihydroxybenzoic Acid is a metabolite of polyphenols such as Phloretin (P339000) and Quercetin (Q509500).
2. A phenolic acid antioxidant and chemopreventive agent
3. 3,4-Dihydroxybenzoic acid is a metabolite of polyphenols such as phloretin and quercetin. It is also a phenolic acid antioxidant present in fruits, vegetables and nuts. Further, it is found to be an efficacious chemopreventive agent in several carcinogenesis models. In addition to this, it acts as an anti-inflammatory agent.
Definition
ChEBI: A dihydroxybenzoic acid in which the hydroxy groups are located at positions 3 and 4.
Biosynthesis
Protocatechuic acid is synthesized from the intermediates of the shikimate pathway. In this case, 3-dehydroshikimate is converted to protocatechuic acid as shown in Fig.11.2. Activity measurements from mung bean seedlings, on the other hand, indicate that 3-dehydroshikimate can be converted to protocatechuic acid (Widhalm and Dudareva 2015). Protocatechuic acid (11.12) upon hydroxylation produces gal- lic acid. The enzyme catalyzing this reaction is not well understood (Muir et al.2011).Biosynthesis of Protocatechuic Acid
General Description
Protocatechuic acid belongs to the class of polyphenolic compounds, which can be generally isolated from the dried flower of roselle. It can serve as a potential candidate possessing anticancer property, which can be involved in suppressing the growth of human promyelocytic leukemia HL-60 cells.
Biological Activity
Protocatechuic acid (PCA) is a dihydroxybenzoic acid phenolic compound found in many edible and medicinal plants. It is a major metabolite of antioxidant polyphenols found in green tea and demonstrates free radical scavenging capability in a 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity assay (IC50 = 16.3 μM). It is thought to possess anti-inflammatory, antihyperglycemic, neuroprotective, and anticancer activities. Dietary administration of PCA dose dependently inhibits in vitro chemical carcinogenesis and exerts pro-apoptotic and anti-proliferative effects in different tissues. In studies of tumor cell migration and invasion using mouse melanoma B16/F10 cells, PCA at 0.1-2 mM down-regulated the Ras/Akt/NF-κB pathway by targeting RhoB activation, leading to a reduction of MMP-mediated activity.
Biochem/physiol Actions
Chemopreventive in several animal models of carcinogenesis. Blocks cell proliferation in the post-initiation phase.
Source
Protocatechuic acid, in free or bound form, is found in the leaves, fruits, wood, barks, flowers of many angiosperms. Free protocatechuic acid is found in the scales of onion (Allium cepa L.), star anise fruits (Illicium L.) and leaves of grapevines (Vitis L.). It is part of the tannins from leaves of apple trees, bark of oak, bark and flowers of acacia (Acacia L). The 3,4-dimethyl ether of protocatechuic acid, the socalled veratric acid, is widely distributed.
Check Digit Verification of cas no
The CAS Registry Mumber 99-50-3 includes 5 digits separated into 3 groups by hyphens. The first part of the number,starting from the left, has 2 digits, 9 and 9 respectively; the second part has 2 digits, 5 and 0 respectively.
Calculate Digit Verification of CAS Registry Number 99-50:
(4*9)+(3*9)+(2*5)+(1*0)=73
73 % 10 = 3
So 99-50-3 is a valid CAS Registry Number.
InChI:InChI=1/C7H6O4/c8-5-2-1-4(7(10)11)3-6(5)9/h1-3,8-9H,(H,10,11)/p-1
99-50-3Relevant articles and documents
Quercetin inhibits advanced glycation end product formation via chelating metal ions, trapping methylglyoxal, and trapping reactive oxygen species
Bhuiyan, Mohammad Nazrul Islam,Mitsuhashi, Shinya,Sigetomi, Kengo,Ubukata, Makoto
, p. 882 - 890 (2017)
Physiological concentration of Mg2+, Cu2+, and Zn2+ accelerated AGE formation only in glucosemediated conditions, which was effectively inhibited by chelating ligands. Only quercetin (10) inhibited MGO-mediated AGE formation as well as glucoseand ribose-mediated AGE formation among 10 polyphenols (1-10) tested. We performed an additional structure-activity relationship (SAR) study on flavanols (10, 11, 12, 13, and 14). Morin (12) and kaempherol (14) showed inhibitory activity against MGO-mediated AGE formation, whereas rutin (11) and fisetin (13) did not. These observations indicate that 3,5,7,4'-tetrahydroxy and 4-keto groups of 10 are important to yield newly revised mono-MGO adducts (16 and 17) and di-MGO adduct (18) having cyclic hemiacetals, while 3'-hydroxy group is not essential. We propose here a comprehensive inhibitory mechanism of 10 against AGE formation including chelation effect, trapping of MGO, and trapping of reactive oxygen species (ROS), which leads to oxidative degradation of 18 to 3,4-dihydroxybenzoic acid (15) and other fragments.
Phenolic compounds from Urtica urens growing in Georgia
Kavtaradze
, p. 314 - 314 (2003)
-
Multi-Enzymatic Cascade Reactions for the Synthesis of cis,cis-Muconic Acid
Di Nardo, Giovanna,Gazzola, Silvia,Gilardi, Gianfranco,Pollegioni, Loredano,Rosini, Elena,Valetti, Francesca,Vignali, Elisa
, p. 114 - 123 (2021/10/07)
Lignin valorization allows the generation of a number of value-added products such as cis,cis-muconic acid (ccMA), which is widely used for the synthesis of chemicals for the production of biodegradable plastic materials. In the present work, we reported the first multi-enzymatic, one-pot bioconversion process of vanillin into ccMA. In details, we used four sequential reactions catalyzed by xanthine oxidase, O-demethylase LigM (and the tetrahydrofolate-regeneration enzyme methyl transferase MetE), decarboxylase AroY (based on the use of E. coli transformed cells) and catechol 1,2-dioxygenase CatA. The optimized lab-scale procedure allowed to reach, for the first time, the conversion of 5 mM vanillin into ccMA in ~30 h with a 90% yield: this achievement represents an improvement in terms of yields and time when compared to the use of a whole-cell system. This multi-enzymatic system represents a sustainable alternative for the production of a high value added product from a renewable resource. (Figure presented.).
Iron-catalyzed arene C-H hydroxylation
Cheng, Lu,Wang, Huihui,Cai, Hengrui,Zhang, Jie,Gong, Xu,Han, Wei
, p. 77 - 81 (2021/10/05)
The sustainable, undirected, and selective catalytic hydroxylation of arenes remains an ongoing research challenge because of the relative inertness of aryl carbon-hydrogen bonds, the higher reactivity of the phenolic products leading to over-oxidized by-products, and the frequently insufficient regioselectivity. We report that iron coordinated by a bioinspired L-cystine-derived ligand can catalyze undirected arene carbon-hydrogen hydroxylation with hydrogen peroxide as the terminal oxidant. The reaction is distinguished by its broad substrate scope, excellent selectivity, and good yields, and it showcases compatibility with oxidation-sensitive functional groups, such as alcohols, polyphenols, aldehydes, and even a boronic acid. This method is well suited for the synthesis of polyphenols through multiple carbon-hydrogen hydroxylations, as well as the late-stage functionalization of natural products and drug molecules.
Ni-NiO heterojunctions: a versatile nanocatalyst for regioselective halogenation and oxidative esterification of aromatics
Bhardwaj, Nivedita,Goel, Bharat,Indra, Arindam,Jain, Shreyans K.,Singh, Ajit Kumar,Tripathi, Nancy
, p. 14177 - 14183 (2021/08/16)
Herein, we report a facile method for the synthesis of Ni-NiO heterojunction nanoparticles, which we utilized for the nuclear halogenation reaction of phenol and substituted phenols usingN-bromosuccinimide (NBS). A remarkablepara-selectivity was achieved for the halogenated products under semi-aqueous conditions. Interestingly, blocking of thepara-position of phenol offeredortho-selective halogenation. In addition, the Ni-NiO nanoparticles catalyzed the oxidative esterification of carbonyl compounds with alcohol, diol or dithiol in the presence of a catalytic amount of NBS. It was observed that the aromatic carbonyls substituted with an electron-donating group favoured nuclear halogenation, whereas an electron-withdrawing group substitution in carbonyl compounds facilitated the oxidation reaction. In addition, the catalyst was magnetically separated and recycled 10 times. The tuned electronic structure at the Ni-NiO heterojunction controlled selectivity and activity as no suchpara-selectivity was observed with commercially available NiO or Ni nanoparticles.