- 2-BROMO-4-(TRIFLUOROMETHYL)PYRIDINE
- 2-Bromo-4-(trifluoromethyl)quinoline
- 2-Bromo-4,6-dimethylpyridine
- 2-Bromo-4,6-dinitroaniline
- 2-Bromo-4,6-diphenylnicotinonitrile
- 2-Bromo-4'-Benzyloxy-3'-nitroacetophenone
- 2-Bromo-4-butanolide
- 2-bromo-4-chloro-6-fluoroaniline
- 2-Bromo-4-chloro-6-nitroaniline
- 2-Bromo-4'-chloroacetophenone
- 18177-89-4Titanate(2-),oxobis[sulfato(2-)-kO,kO']-, disodium (9CI)
- 1817-90-9Benzene,(2-ethoxyethyl)-
- 18179-39-0Benzoic acid,2-hydroxy-4-iodo-, methyl ester
- 18179-67-4Benzenesulfonamide,4-amino-N-(5-methoxy-2-pyrimidinyl)-, sodium salt (1:1)
- 181801-29-6Pyrimidine, 2-(4-nitrophenoxy)-
- 18180-29-52-Propanol,1-chloro-3-(2-propyn-1-yloxy)-
- 18180-30-8Oxirane,2-[(2-propyn-1-yloxy)methyl]-
- 181816-48-8Propanamide,2-amino-3-hydroxy-N-[2-methoxy-5-[(1Z)-2-(3,4,5-trimethoxyphenyl)ethenyl]phenyl]-,(2S)-
- 18181-80-1Benzeneacetic acid,4-bromo-a-(4-bromophenyl)-a-hydroxy-, 1-methylethyl ester
- 18182-14-4Silane,methoxydimethylpropyl-
Hot Products
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
- 7631-86-9Silicon dioxide
- 302-79-4Tretinoin
- 77-92-9Citric acid

|
Basic Information |

|
Post buying leads |

|
Suppliers |

| Name |
2-Bromo-4,6-dinitroaniline |
EINECS | 217-329-2 |
| CAS No. | 1817-73-8 | Density | 1.991 g/cm3 |
| PSA | 117.66000 | LogP | 3.47530 |
| Solubility | N/A | Melting Point |
151-153 °C(lit.) |
| Formula | C6H4BrN3O4 | Boiling Point | 400.8 °C at 760 mmHg |
| Molecular Weight | 262.019 | Flash Point | 196.2 °C |
| Transport Information | N/A | Appearance | Yellow to yellow brown powder |
| Safety | 37/39-26-16 | Risk Codes | 36/37/38-11 |
| Molecular Structure |
|
Hazard Symbols |
 Xi, Xi,  F F
|
| Synonyms |
Aniline,2-bromo-4,6-dinitro- (6CI,7CI,8CI);2,4-Dinitro-6-bromoaniline;2-Bromo-4,6-dinitroaminobenzene;6-Bromo-2,4-dinitroaniline;NSC 16572; |
2-Bromo-4,6-dinitroaniline Chemical Properties
Chemical Name: 2-Bromo-4,6-dinitroaniline
IUPAC NAME: 2-bromo-4,6-dinitroaniline
CAS No.: 1817-73-8
EINECS: 217-329-2
RTECS: BW9303000
RTECS Class: Mutagen; Primary Irritant
Molecular Formula: C6H4BrN3O4
Molecular Weight: 262.02 g/mol
Density: 1.991 g/cm3
Melting Point: 151-153 °C(lit.)
Flash Point: 196.2 °C
Boiling Point: 400.8 °C at 760 mmHg
2,4-DINITRO-6-BROMOANILINE(1817-73-8) incompatible with xoidizing agents,strong bases.Following is the structure of 2,4-DINITRO-6-BROMOANILINE(1817-73-8):
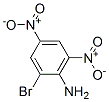
The chemical synonymous of 2,4-DINITRO-6-BROMOANILINE(1817-73-8) are 2,4-dinitro-6-bromanilin;2,4-dinitro-6-bromoanilin(czech);2-bromo-4,6-dinitro-anilin;2-bromo-4,6-dinitro-benzenamid;2-bromo-4,6-dinitro-benzenamin;bromodna;nci-c60844;2,4-DINITRO-6-BROMO ANILINE.
2-Bromo-4,6-dinitroaniline Uses
2-Bromo-4,6-dinitroaniline Production
Product Categories about 2,4-DINITRO-6-BROMOANILINE(1817-73-8) are Intermediates of Dyes and Pigments;Amines;C2 to C6;Nitrogen Compounds.
2-Bromo-4,6-dinitroaniline Toxicity Data With Reference
| 1. | eye-rbt 500 mg/24H SEV | 28ZPAK Sbornik Vysledku Toxixologickeho Vysetreni Latek A Pripravku Marhold, J.V.,Institut Pro Vychovu Vedoucicn Pracovniku Chemickeho Prumyclu Praha,Czechoslovakia.: 1972,94. | ||
| 2. | mmo-sat 10 µg/plate | ENMUDM Environmental Mutagenesis. 9 (Suppl 9)(1987),1. | ||
| 3. | orl-rat LD50:4100 mg/kg | TSCAT* Office of Toxic Substances Report. (U.S. Environmental Protection Agency, Office of Toxics Substances, 401M Street SW, Washington, DC 20460) OTS 206481 . |
| rat | LD50 | oral | 4100mg/kg (4100mg/kg) | BRAIN AND COVERINGS: RECORDINGS FROM SPECIFIC AREAS OF CNS BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | Office of Toxic Substances Report. Vol. OTS, |
2-Bromo-4,6-dinitroaniline Consensus Reports
2-Bromo-4,6-dinitroaniline Safety Profile
Mildly toxic by ingestion. A severe eye irritant. Mutation data reported. When heated to decomposition it emits very toxic fumes of Br− and NOx. See also 2,4-DINITROANILINE.
Hazard Codes:
Xi: Harmful 
F: Flammable 
Risk Statements about 2,4-DINITRO-6-BROMOANILINE(1817-73-8):
R11 Highly flammable.
R36/37/38: Irritating to eyes, respiratory system and skin.
Safety Statements about 2,4-DINITRO-6-BROMOANILINE(1817-73-8):
S16 Keep away from sources of ignition.
S26 In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S37/39: Wear suitable gloves and eye/face protection.
2-Bromo-4,6-dinitroaniline Specification
1. Storage: Store in a cool, dry, well-ventilated area away from incompatible substances. Keep containers tightly closed.
2. Handling: Avoid contact with eyes, skin, and clothing. Minimize dust generation and accumulation. Keep container tightly closed. Use with adequate ventilation.
3. Personal Protection: Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166. Skin: Wear appropriate protective gloves to prevent skin exposure. Clothing: Wear appropriate protective clothing to prevent skin exposure.
4. Fire Fighting: Wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. To extinguish fire, use carbon dioxide, dry chemical powder or appropriate foam.
5. Reactivity Profile: A halogenated and nitrated amine. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides.

