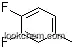
2927-34-6Relevant articles and documents
Two-step regioselective synthesis of 1,2-difluorobenzenes from chlorotrifluoroethylene and buta-l,3-dienes
Lipkind, M. B.,Nefedov, O. M.,Volchkov, N. V.
, p. 68 - 75 (2020/04/21)
The gas-phase copyrolysis of chlorotrifluoroethylene with buta-1,3-diene, penta-1,3-diene, or isoprene in a flow reactor at 440–480°C gave 4-chloro-4,5,5-trifluorocyclohex-1-enes. The latter treated with aqueous KOH under condition of phase-transfer catalysis were selectively converted into 1,2-difluorobenzene, 2,3-difluorotoluene, or 3,4-difluorotoluene.
LIQUID CRYSTALLINE COMPOUND HAVING DIBENZOTHIOPHENE RING, LIQUID CRYSTAL COMPOSITION AND LIQUID CRYSTAL DISPLAY ELEMENT
-
Paragraph 0167-0168, (2020/10/27)
PROBLEM TO BE SOLVED: To provide a liquid crystalline compound satisfying at least one of physical properties such as high stability to heat or light, a high clear point, a low minimum temperature of a liquid crystal phase, small viscosity, appropriate optical anisotropy, negatively large dielectric anisotropy, an appropriate elastic constant and good compatibility with other liquid crystalline compounds, a liquid crystal composition containing the compound, and a liquid crystal display element containing the composition. SOLUTION: The compound is represented by formula (1), and the liquid crystal composition contains the above compound. In the formula, R1 and R2 represent an alkyl having 1 to 16 carbon atoms, or the like; A1 to A2 represent 1,4-cyclohexylene or the like; Z1 and Z2 represent a single bond or the like; m1 and n1 represent 0, 1 or 2; W represents -S- or the like; X represents H or F; and Y1 to Y4 represent H or a methyl. SELECTED DRAWING: None COPYRIGHT: (C)2021,JPOandINPIT
Method of preparing fluoroaromatic compounds
-
, (2008/06/13)
The present invention provides a method of preparing ortho-difluorobenzene derivatives, which comprises (a) providing a mixture of cyclohexenes by reacting chlorotrifluoroethylene (CTFE) and 1,3-diene in a flow reactor and distilling the resultant, and (b) dehydrohalogenating the mixture of cyclohexenes with a phase transition catalyst in the presence of alkali metal hydroxide at temperature range of 40 to 150 °C without using any organic solvent. The distillate having low boiling point, which is obtained during distillation of the resultant, is recycled into the flow reactor. The present invention also provides a method of preparing 2-chloro-4,5-difluorobenzoic acid, which comprises (a) providing a mixture of 4-chloro-1-methyl-4,5,5-trifluorocyclohexene and 5-chloro-1-methyl-4,4,5-trifluorocyclohexene by reacting chlorotrifluoroethylene (CTFE) and isoprene and distilling the resultant, (b) dehydrohalogenating said mixture in the presence of alkali metal hydroxide and a phase transition catalyst to form 3,4-difluorotoluene, (c) reacting said 3,4-difluorotoluene with chlorine gas without using any organic solvent to form 2-chloro-4,5-difluorotoluene, (d) photo-reacting said 2-chloro-4,5-difluorotoluene with chlorine gas under a lighting mercury lamp without using any organic solvent to form 2-chloro-4,5-difluorobenzotrichloride, and (e) reacting said 2-chloro-4,5-difluorobenzotrichloride with aqueous acid solution without using any organic solvent. The present invention also provides a method of preparing 2-chloro-4,5-difluorobenzoyl chloride by reacting the 2-chloro-4,5-difluorobenzotrichloride of step (e) above with zinc oxide.