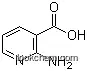
5345-47-1Relevant articles and documents
Preparation method of 2-aminopyridine-3-carboxylic acid
-
Paragraph 0015-0029, (2022/01/12)
The present invention discloses a method for preparing 2- aminopyridine-3-carboxylic acid, comprising the following steps: adding 500g of deionized water in a 1L three-mouth bottle, adding raw material 2-amino-3-methylpyridine 100g under stirring, adding a catalyst of 186g and heating to 55-65 ° C, and then controlling the temperature at 60-70 ° C in batches to add potassium permanganate 146g, after addition continue stirring reaction for 3 hours; then cooled to room temperature, filtered to remove black manganese dioxide and recovered After adjusting the PH to 2.5 in the aqueous phase, freezing to 3-7 ° C filtration, the white filter cake was obtained, and then washed and dried in ice water to give the product 2-amino 2-aminopyridine-3-carboxylic acid 65 grams. The preparation method of the intermediate 2-aminopyridine-3-carboxylic acid of this drug has reasonable design of the whole process, mild conditions, simple operation, easy raw materials and high generation efficiency. The reaction of this method is safe and controllable, and the obtained manganese dioxide by-products can be recycled, and the yield is high, which is conducive to industrial production.
Copper(ii)-catalyzed c-n coupling of aryl halides and n-nucleophiles promoted by quebrachitol or diethylene glycol
Chen, Guoliang,Chen, Yuanguang,Du, Fangyu,Fu, Yang,Wu, Ying,Zhou, Qifan
supporting information, p. 2161 - 2168 (2019/11/25)
Herein, we report the natural ligand quebrachitol (QCT) as a promoter for a Cu(II) catalyst, which is highly effective for N-Arylation of various amines and related aryl halides. A series of diarylamine derivatives were obtained in high yields by using diethylene glycol (DEG) as both ligand and solvent. The C-N coupling reactions proceed under mild conditions and exhibit good functional group tolerance.
Heterocyclic mutilin esters and their use as antibacterials
-
, (2008/06/13)
Pleuromutilin compounds of the formula: 1are of use in anti-bacterial therapy.