- 308076-80-4Synthetic fibers,yttrium-stabilized zirconium oxide
- 308080-99-1Zeolite3A
- 308103-40-42-Acetylphenylboronic acid
- 3081-14-91,4-Benzenediamine,N1,N4-bis(1,4-dimethylpentyl)-
- 30811-80-45'-Cytidylic acid,homopolymer
- 3081-61-6L-Glutamine,N-ethyl-
- 30818-11-2Ethanone, 1-(4-piperidinyl)-
- 30818-17-83H-Pyrazol-3-one,2,4-dihydro-2-(4-nitrophenyl)-5-(1-pyrrolidinyl)-
- 30818-18-9Butanamide,2-[2,4-bis(1,1-dimethylpropyl)phenoxy]-N-[4-[4,5-dihydro-5-oxo-4-[(1-phenyl-1H-tetrazol-5-yl)thio]-3-(1-pyrrolidinyl)-1H-pyrazol-1-yl]phenyl]-
- 30820-22-5Cyclobutaneethanol,1-methyl-2-(1-methylethenyl)-, (1R,2S)-rel-
Hot Products
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
- 7631-86-9Silicon dioxide
- 302-79-4Tretinoin
- 77-92-9Citric acid

|
Basic Information |

|
Post buying leads |

|
Suppliers |

| Name |
GLASSY CARBON |
EINECS | 231-153-3 |
| CAS No. | 308068-56-6 | Density | ~1.7 |
| PSA | 0.00000 | LogP | 0.00000 |
| Solubility | It is miscible, can be diluted with water. Soluble in benzene, toluene. | Melting Point |
3550 °C(lit.) |
| Formula | C | Boiling Point |
500-600 °C(lit.) |
| Molecular Weight | 12.01 | Flash Point | >230 |
| Transport Information | N/A | Appearance | Powder |
| Safety | 26-36-61-37/39-22 | Risk Codes | 36/37-36/37/38-52/53 |
| Molecular Structure |
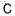
|
Hazard Symbols | F,Xi,N |
| Synonyms |
VGCF-G;Tubulenes;Tubulene;Tubularfullerenes;Carbon nanotubes;Sunnano SWNT;SWCNT;Carbon nanotube;CNT;Fullerenes,tubular; |
GLASSY CARBON Chemical Properties
Glassy CARBON, can called vitreous CARBON too, is a non-graphitizing CARBON which combines glassy and ceramic properties with those of graphite. The most important properties are high temperature resistance, extreme resistance to chemical attack and impermeability to gases and liquids. Glassy CARBON is widely used as an electrode material in electrochemistry, as well as for high temperature crucibles and as a component of some prosthetic devices.
GLASSY CARBON History
Glassy carbon was first produced by workers at the laboratories of The General Electric Company, UK, in the early 1960s, using cellulose as the starting material. A short time later, Japanese workers produced a similar material from phenolic resin. The preparation of glassy carbon involves subjecting the organic precursors to a series of heat treatments at temperatures up to 3000 °C.
Glassy carbon was first observed in the laboratories of The Carborundum Company, Trafford Park, Manchester, UK, in the mid 1950's by Bernard Redfern (BR) the inventor, a materials scientist and diamond technologist.
GLASSY CARBON Uses
1. in rubber industry for the manufacture of natural rubber and butyl rubber reinforcing agent and filler, can give the vulcanized rubber with good tensile strength, elongation and wear resistance etc.. The utility model is mainly used for the large tread of natural rubber and the tread of various cross-country tyres, and can also be used for carcass and sidewall, and can also be used for high strength conveyer belts, cold resistant rubber products and drilling parts. Packing light industry for paint, ink, enamel and plastic products.
2.. Used as a black colorant for plastics, rubber, ink, paper, etc.. Used in the manufacture of eye and eyebrow pencil in cosmetics.
GLASSY CARBON Specification
Note that glassy carbon should not be confused with amorphous carbon. This from IUPAC: "Glass-like carbon cannot be described as amorphous carbon because it consists of two-dimensional structural elements and does not exhibit ‘dangling’ bonds."
Using this phenolic resin, crucibles were produced. Crucibles were distributed to organisations such as UKAEA Harwell. BR left the Carborundom Co., which officially wrote off all interests in the glassy carbon invention. Whilst working at the Plessey Company laboratory (a disused church!) in Towcester, UK, BR received a glassy carbon crucible for duplication from UKAEA. He identified it as one he had made from markings he had engraved into the uncured precursor prior to carbonisation. (It is almost impossible to engrave the finished product.) The Plessey Company set up a laboratory first in a factory previously used to make briar pipes, in Litchborough, UK, and then a permanent facility at Caswell, near Blakesly, UK. Caswell became the Plessey Research centre and then the Alan Clark research Centre. Glassy carbon arrived at the Plessey Company Limited as a fait accompli. BR was assigned two associates for the production of glassy carbon. F. C. Cowlard was an administrator who previously had some association with Silane and J. C. Lewis was a chemical assistant. Large sections of the precursor material were produced as castings or machined into a predetermined shape. Large crucibles and other forms were manufactured. Carbonisation took place in two stages. Shrinkage during this process is considerable but absolutely uniform and predictable. Some of the first ultrapure samples of Gallium Arsenide were zone refined in these crucibles. (Glassy carbon is extremely pure and unreactive to GaAs). Patents were filed and the name "Vitreous Carbon" presented to the product by the son of BR. Glassy/Vitreous Carbon was under investigation used for components for thermonuclear detonation systems and at least some of the patents surrounding the material were rescinded (in the interests of national security) in the 1960s.

