Lead(ii) thiocyanate Synthetic route
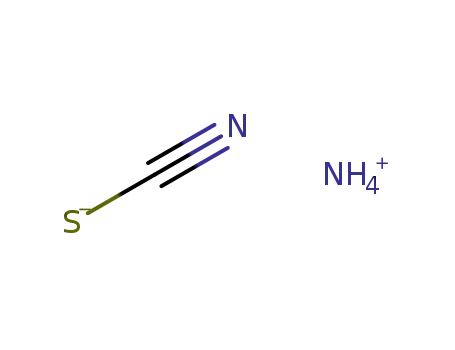
ammonium thiocyanate
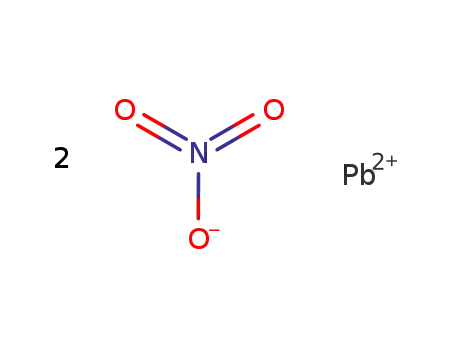
lead(II) nitrate
Conditions
| Conditions | Yield |
|---|
| In water addn. of 1M aq. Pb(NO3)2 soln. to 136ml 10M NH4SCN soln. at 5°C, stirring for 30min; (exclusion of light);; washing with H2O of 5°C, drying in vac. over P2O5 for about 8d; (exclusion of light);; | 97% |
| In water addn. of 1M aq. Pb(NO3)2 soln. to 136ml 10M NH4SCN soln. at 5°C, stirring for 30min; (exclusion of light);; washing with H2O of 5°C, drying in vac. over P2O5 for about 8d; (exclusion of light);; | 97% |
| In water byproducts: Pb3{Bi(SCN)6}2; pptn. from Bi containing solns.;; product mixture;; | |
| In water NH4SCN soln. was added to aq. Pb-salt soln. in stoich. amt.; ppt. was filtered, washed with water, dried in an oven at 105 °C; | |
| In water byproducts: Pb3{Bi(SCN)6}2; pptn. from Bi containing solns.;; product mixture;; | |
Conditions
| Conditions | Yield |
|---|
| With lead(II) nitrate; water at 0 - 5℃; | |
Conditions
| Conditions | Yield |
|---|
| With lead(II) nitrate; water at 5℃; | |

ammonium thiocyanate
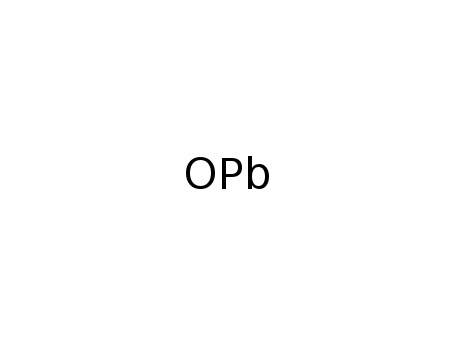
lead(II) oxide
A
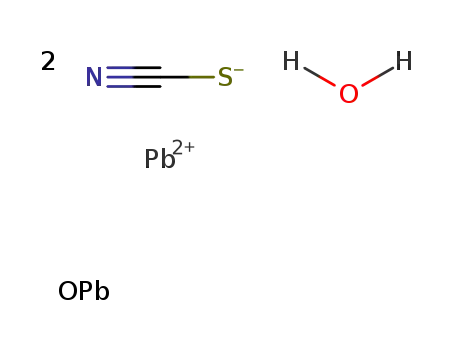
thiocyanic acid ; lead (II)-compound with lead (II)-oxide
Conditions
| Conditions | Yield |
|---|
| In acetonitrile elem. anal.;; | |
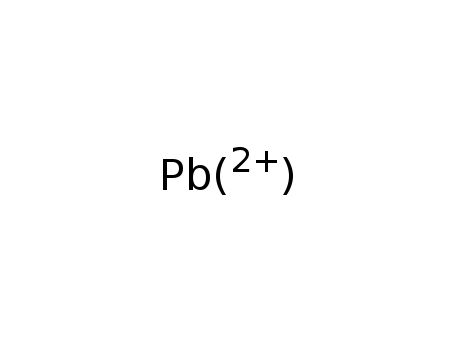
lead(2+) cation
A
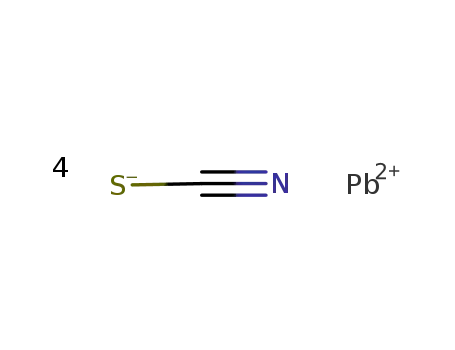
Pb(2+)*4SCN(1-)=Pb(SCN)4(2-)
B
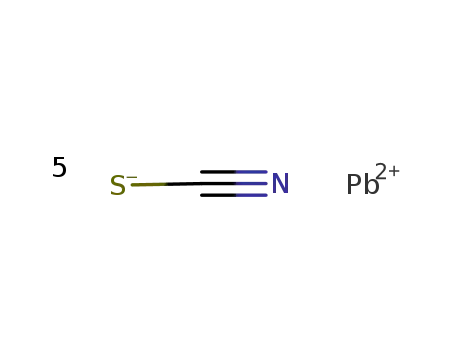
Pb(2+)*5SCN(1-)=Pb(SCN)5(3-)
C
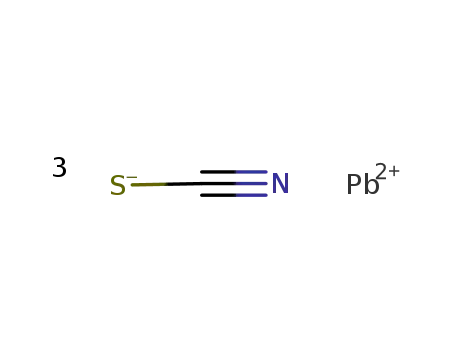
Pb(2+)*3SCN(1-)=Pb(SCN)3(1-)
Conditions
| Conditions | Yield |
|---|
| In water 4 - 10 M KSCN;; | |
| In water 4 - 10 M KSCN;; | |
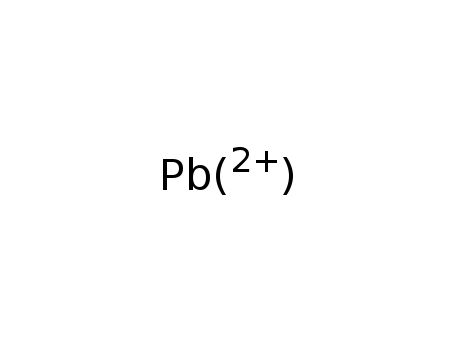
lead(2+) cation
Conditions
| Conditions | Yield |
|---|
| In water pptn.;; | |
| In water pptn.;; | |
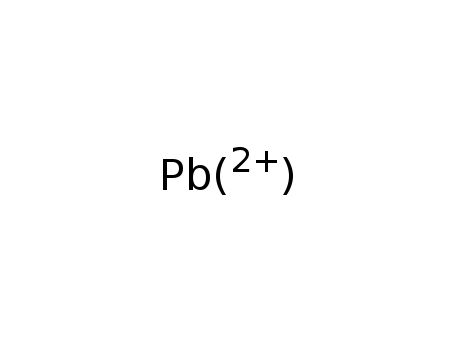
lead(2+) cation
A
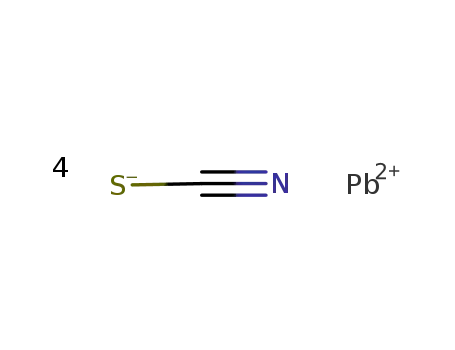
Pb(2+)*4SCN(1-)=Pb(SCN)4(2-)
B
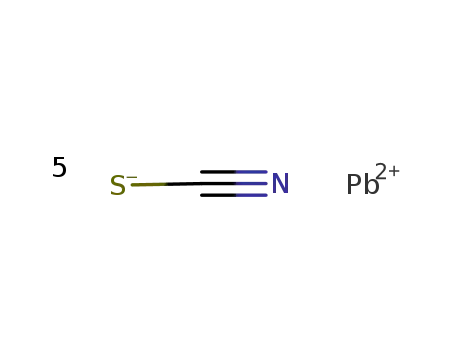
Pb(2+)*5SCN(1-)=Pb(SCN)5(3-)
C
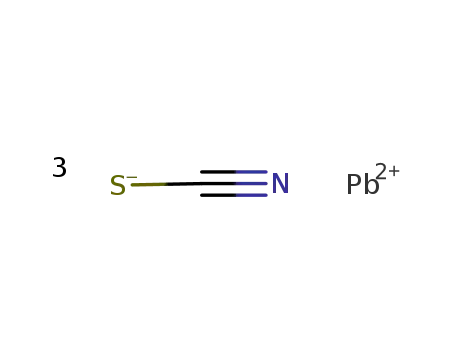
Pb(2+)*3SCN(1-)=Pb(SCN)3(1-)
Conditions
| Conditions | Yield |
|---|
| In water 3.6 - 5.87 M NaSCN;; | |
| In water 3.6 - 5.87 M NaSCN;; | |

lead(II) sulfide
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) with PbS on clay in HSCN atmosphere >150°C;; | |
| In neat (no solvent) with PbS on clay in HSCN atmosphere >150°C;; | |
Lead(ii) thiocyanate Chemical Properties
Chemical Name: Lead(ii) thiocyanate
IUPAC NAME: Lead(2+) dithiocyanate
CAS No.: 592-87-0
EINECS: 209-774-6
Molecular Formula: C2N2PbS2
Molecular Weight: 323.36 g/mol
Melting Point: 190 °C (dec.)(lit.)
Density: 3.82 g/mL at 25 °C(lit.)
Flash Point: 42.1 °C
Boiling Point: 146 °C at 760 mmHg
Following is the structure of Lead(ii) thiocyanate (592-87-0):

The chemical synonymous of Lead(ii) thiocyanate (592-87-0) are Lead thiocyanate ; Lead sulfocyanide ; Lead(Ii) rhodanide ; Lead(Ii) thiocyanate ; Plumbous thiocyanate ; Isothiocyanicacid,Lead(2+)salt ; Lead(Ii)thiocyanate(Pb(Ncs)2) ; Leaddithiocyanate
Lead(ii) thiocyanate Consensus Reports
Lead and its compounds are on the Community Right-To-Know List.
Lead(ii) thiocyanate Safety Profile
An explosive. When heated to decomposition it emits toxic fumes of Pb, SOx, and NOx. See also LEAD COMPOUNDS and THIOCYANATES.
Hazard Codes:
T: Toxic .gif)
N: Dangerous for the environment .gif)
Risk Statements about Lead(ii) thiocyanate (592-87-0):
R32 Contact with acid liberates very toxic gas.
R33 Danger of cumulative effects.
R61 May cause harm to the unborn child.
R62 Risk of impaired fertility.
R20/21/22: Harmful by inhalation, in contact with skin and if swallowed.
R50/53: Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment.
Safety Statements about Lead(ii) thiocyanate (592-87-0):
S13 Keep away from food, drink and animal foodstuffs.
S45 In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S53 Avoid exposure - obtain special instructions before use.
S60 This material and its container must be disposed of as hazardous waste.
S61 Avoid release to the environment. Refer to special instructions / safety data sheets.
Health Hazard: Early symptoms of lead intoxication via inhalation or ingestion are most commonly gastrointestinal disorders, colic, constipation, etc. ; weakness, which may go on to paralysis, chiefly of the extensor muscles of the wrists and less often of the ankles, is noticeable in the most serious cases. Ingestion of a laarge amount causes local irritation of the alimentary tract ; pain, leg cramps, muscle weakness, paresthesias, depression, coma, and death may follow in 1 or 2 days. Contact causes irritation of eyes and mild irritation of skin.
Lead(ii) thiocyanate Standards and Recommendations
ACGIH TLV: TWA 0.15 mg(Pb)/m3
Lead(ii) thiocyanate Specification
Lead(ii) thiocyanate (592-87-0) is a chemical compound, more precisely a salt, with the chemical formula Pb(SCN)2.It may be made by reacting lead(II) acetate (Pb(CH3COO)2) solved in water with either potassium thiocyanate (KSCN) or ammonium thiocyanate (NH4SCN), thus causing precipitation of solid lead(II) thiocyanate.
Ion reaction as follows:
Pb2+(aq) + 2SCN-(aq) → Pb(SCN)2(s)
It is reasonably soluble at room temperature, thus it may be difficult to identify in a solution with low concentration of lead(II) thiocyanate. Although it has not been confirmed by other sources than the author of this article, experiments show that even if there is no precipitation of lead(II) thiocyanate in the solution, crystals of the salt may form.





 T,
T,  N
N














![]()
![]()

