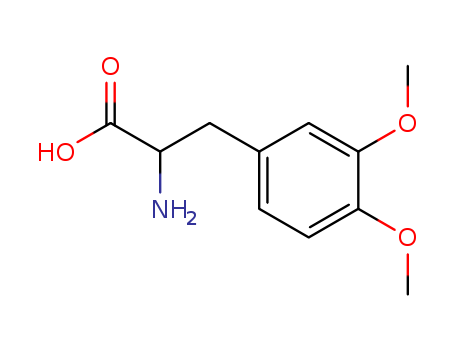
- Chemical Name:2-Amino-3-(3,4-dimethoxyphenyl)propanoic acid
- CAS No.:55-59-4
- Molecular Formula:C11H15NO4
- Molecular Weight:225.244
- Hs Code.:2922509090
- NSC Number:121994
- UNII:OV6Z38VT4R
- DSSTox Substance ID:DTXSID901018930
- Wikidata:Q27285857
- Mol file:55-59-4.mol
Synonyms:55-59-4;2-amino-3-(3,4-dimethoxyphenyl)propanoic acid;3,4-Dimethoxyphenylalanine;DL-3,4-Dimethoxyphenylalanine;3,4-Dimethoxyphenylalanine, DL-;3,4-Dimethoxy-dl-phenylalanine;UNII-OV6Z38VT4R;OV6Z38VT4R;Tyrosine, 3-methoxy-O-methyl-;MFCD00037201;NSC-121994;Tyrosine,3-methoxy-O-methyl-;NSC121994;S-3,4-DIMETHOXY-PHENYLALANINE;3,4-Dimethoxyphenylalanine #;SCHEMBL159000;2-AMINO-3-(3,4-DIMETHOXY-PHENYL)-PROPIONIC ACID;VWTFNYVAFGYEKI-UHFFFAOYSA-N;DTXSID901018930;2-Methyl-5-nitrobenzenesulfonicacid;AKOS000180195;AKOS022183305;AB01633;AB33629;DS-17334;SY044907;CS-0046967;FT-0613603;FT-0624392;FT-0631243;W16159;EN300-1844390;Q27285857;2-Amino-3-(3,4-dimethoxyphenyl)propanoic acid (H-DL-Phe(3,4-diOMe)-OH)


 Xi
Xi

