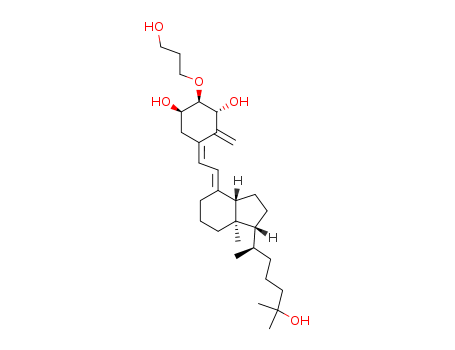
- Chemical Name:Eldecalcitol
- CAS No.:104121-92-8
- Molecular Formula:C30H50O5
- Molecular Weight:490.724
- Hs Code.:
- UNII:I2JP8UE90H
- Wikipedia:Eldecalcitol
- NCI Thesaurus Code:C87291
- Pharos Ligand ID:UTYAZWAWL8XU
- Metabolomics Workbench ID:36020
- ChEMBL ID:CHEMBL4297608
- Mol file:104121-92-8.mol
Synonyms:2-(3-hydroxypropoxy)-1,25-dihydroxyvitamin D3;2-(3-hydroxypropoxy)calcitriol;20-epi-1alpha,25-dihydroxy-2beta-(3-hydroxypropoxy)vitamin D3;20-epi-ED-71;20-epi-eldecalcitol;ED 71;ED-71;eldecalcitol