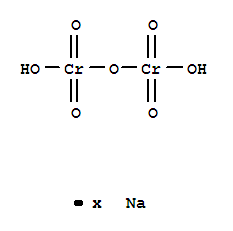Base Information
Edit
- Chemical Name:Sodium Dichromate
- CAS No.:34493-01-1
- Deprecated CAS:12018-32-5,1018900-84-9,1088355-13-8,1391351-00-0,1018900-84-9,1391351-00-0
- Molecular Formula:Cr2H2 O7 . x Na
- Molecular Weight:261.96754
- Hs Code.:
- European Community (EC) Number:234-190-3
- ICSC Number:1369
- UN Number:3087,3288,1479
- UNII:C9G6VY6ZZ4
- DSSTox Substance ID:DTXSID8021274
- Wikipedia:Sodium dichromate dihydrate,Sodium dichromate,Sodium_dichromate
- Wikidata:Q407873
- NCI Thesaurus Code:C45888
- Mol file:34493-01-1.mol
Synonyms:Na2Cr2O7;sodium bichromate;sodium bichromate, dihydrate;sodium bichromate, monohydrate;sodium bichromate, x-Na salt;sodium dichromate