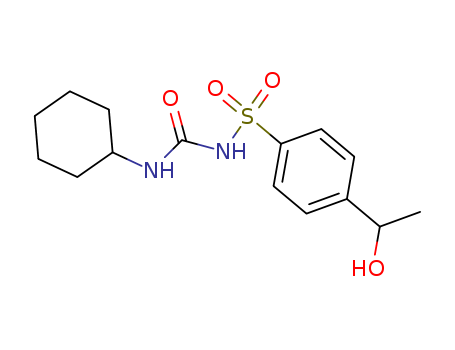
- Chemical Name:Hydroxyhexamide
- CAS No.:3168-01-2
- Molecular Formula:C15H22 N2 O4 S
- Molecular Weight:326.417
- Hs Code.:2935009090
- UNII:F3F26TZ9HN
- DSSTox Substance ID:DTXSID50953633
- Nikkaji Number:J9.524I
- Wikidata:Q27277583
- ChEMBL ID:CHEMBL2105052
- Mol file:3168-01-2.mol
Synonyms:hydroxyhexamide;hydroxyhexamide, (+-)-isomer;hydroxyhexamide, (S)-isomer