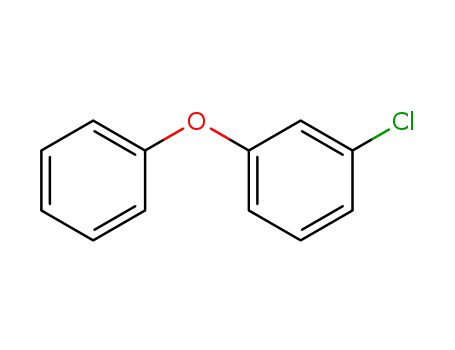
- Chemical Name:1-Chloro-3-phenoxybenzene
- CAS No.:6452-49-9
- Molecular Formula:C12H9 Cl O
- Molecular Weight:204.656
- Hs Code.:
- European Community (EC) Number:229-254-2
- UNII:R33083ZT00
- DSSTox Substance ID:DTXSID1064370
- Nikkaji Number:J127.126A
- Wikidata:Q27287720
- Mol file:6452-49-9.mol
Synonyms:1-Chloro-3-phenoxybenzene;3-CHLORODIPHENYL ETHER;6452-49-9;Benzene, 1-chloro-3-phenoxy-;Ether, m-chlorophenyl phenyl;m-Chlorophenyl phenyl ether;1-chloro-3-phenoxy-benzene;UNII-R33083ZT00;EINECS 229-254-2;R33083ZT00;3-chlorophenyl phenyl ether;PCDE 2;3-PHENOXYCHLOROBENZENE;1-Chloro-3-phenoxybenzene #;SCHEMBL3790523;DTXSID1064370;BMURONZFJJPAOK-UHFFFAOYSA-;Q27287720