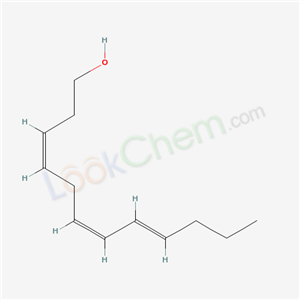
- Chemical Name:3Z,6Z,8E-Dodecatrien-1-ol
- CAS No.:19926-64-8
- Molecular Formula:C12H20O
- Molecular Weight:180.2866
- Hs Code.:
- Metabolomics Workbench ID:3256
- Mol file:19926-64-8.mol
Synonyms:3Z,6Z,8E-Dodecatrien-1-ol;19926-64-8;(3Z,6Z,8E)-dodeca-3,6,8-trien-1-ol;(z,e,e)-3,6,8-dodecatrien-1-ol;3,6,8-Dodecatrien-1-ol, (3Z,6Z,8E)-;(z,z,e)-3,6,8-dodecatrien-1-ol;SCHEMBL1300871;LMFA05000166