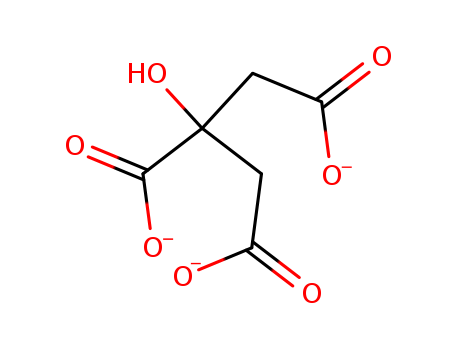
- Chemical Name:Citrate
- CAS No.:126-44-3
- Molecular Formula:C6H5 O7
- Molecular Weight:189.101
- Hs Code.:
- UNII:664CCH53PI
- DSSTox Substance ID:DTXSID30155037
- Nikkaji Number:J209.358H
- Wikipedia:Citrate ion
- Wikidata:Q55503059
- NCI Thesaurus Code:C63374
- Mol file:126-44-3.mol
Synonyms:Anhydrous Citric Acid;Citrate;Citric Acid;Citric Acid Monohydrate;Citric Acid, Anhydrous;Uralyt U