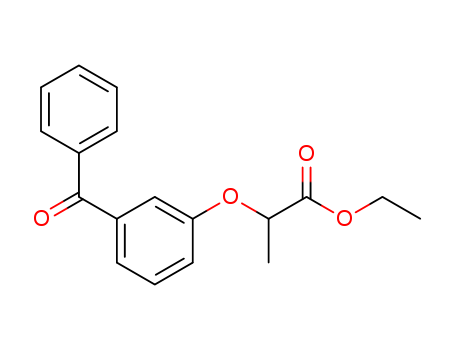
- Chemical Name:2-(m-Benzoylphenoxy)propionic acid ethyl ester
- CAS No.:74167-91-2
- Molecular Formula:C18H18O4
- Molecular Weight:298.339
- Hs Code.:
- DSSTox Substance ID:DTXSID00995629
- Nikkaji Number:J92.634E
- Mol file:74167-91-2.mol
Synonyms:2-(m-Benzoylphenoxy)propionic acid ethyl ester;74167-91-2;BRN 5572864;Propionic acid, 2-(m-benzoylphenoxy)-, ethyl ester;ethyl 2-(3-benzoylphenoxy)propanoate;DTXSID00995629;LS-124504