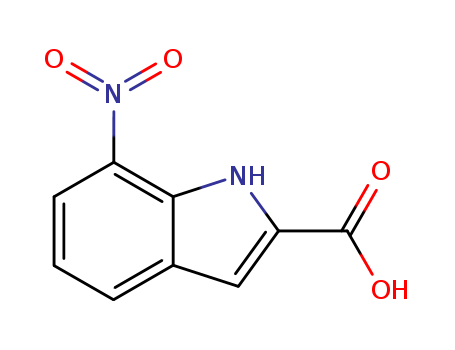
- Chemical Name:7-Nitroindole-2-carboxylic acid
- CAS No.:6960-45-8
- Molecular Formula:C9H6N2O4
- Molecular Weight:206.158
- Hs Code.:2933990090
- European Community (EC) Number:230-154-6
- NSC Number:69877
- DSSTox Substance ID:DTXSID7064520
- Nikkaji Number:J266.002D
- Wikidata:Q72494862
- ChEMBL ID:CHEMBL1870764
- Mol file:6960-45-8.mol
Synonyms:7-nitro-1H-indole-2-carboxylic acid;CRT 0044876;CRT-0044876;CRT0044876