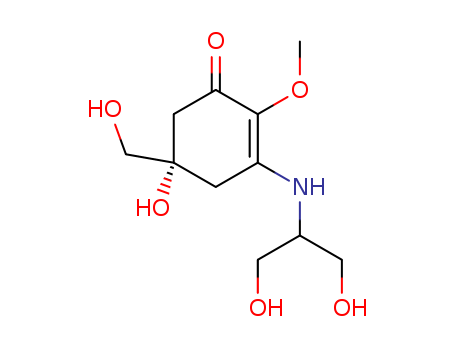
- Chemical Name:Mycosporine
- CAS No.:59719-29-8
- Molecular Formula:C11H19NO6
- Molecular Weight:261.27
- Hs Code.:
- DSSTox Substance ID:DTXSID00331982
- Nikkaji Number:J667.925K
- Wikidata:Q27107431
- Metabolomics Workbench ID:69478
- ChEMBL ID:CHEMBL1210424
- Mol file:59719-29-8.mol
Synonyms:Mycosporine;Mycosporine Serinol;59719-29-8;CHEBI:7039;CHEMBL1210424;C10607;(5S)-3-(1,3-dihydroxypropan-2-ylamino)-5-hydroxy-5-(hydroxymethyl)-2-methoxycyclohex-2-en-1-one;AC1L9DJE;DTXSID00331982;Q27107431;(5S)-5-hydroxy-3-[[2-hydroxy-1-(hydroxymethyl)ethyl]amino]-5-(hydroxymethyl)-2-methoxy-cyclohex-2-en-1-one