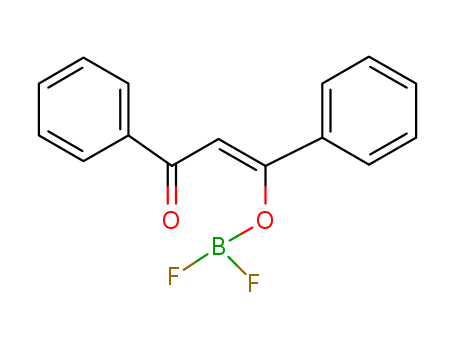
- Chemical Name:Boron dibenzoylmethane difluoride
- CAS No.:397-85-3
- Molecular Formula:C15H11BF2O2
- Molecular Weight:272.0544
- Hs Code.:
- NSC Number:103201
- Nikkaji Number:J3.300.351J
- Mol file:397-85-3.mol
Synonyms:Boron dibenzoylmethane difluoride;NSC103201;397-85-3;Dibenzoylmethaneboron difluoride;SCHEMBL15189219;SGGDSTGSFUIPBJ-PTNGSMBKSA-N;(Dibenzoylmethanoato)boron difluoride;NSC-103201;(1,3-Diphenyl-1,3-propanedionato)difluoroboron;3-Oxo-1,3-diphenyl-1-propenyl difluoridoborate #;Boron, (1,3-diphenyl-1,3-propanedionato)difluoro-;Boron, difluoro(1,3-diphenyl-1,3-propanedionato)-;(Z)-3-(Difluoroboryloxy)-1,3-diphenyl-2-propene-1-one;Boron, (1,3-diphenyl-1,3-propanedioato-o,o')difluoro-, (t-4)-