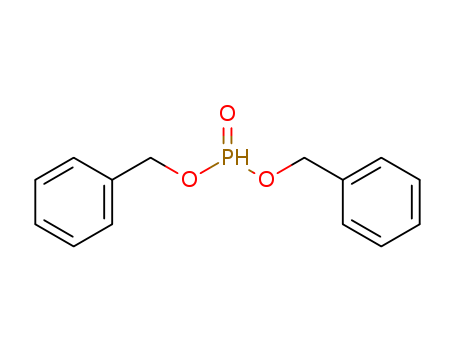
- Chemical Name:Dibenzyl phosphite
- CAS No.:17176-77-1
- Molecular Formula:C14H15O3P
- Molecular Weight:262.245
- Hs Code.:29209090
- European Community (EC) Number:241-226-1
- UNII:1O720L5H5A
- DSSTox Substance ID:DTXSID20938038
- Wikidata:Q27252680
- Mol file:17176-77-1.mol
Synonyms:Dibenzyl phosphite;Dibenzyl phosphonate;17176-77-1;Phosphonic acid dibenzyl ester;oxo-bis(phenylmethoxy)phosphanium;Phosphonic acid, bis(phenylmethyl) ester;MFCD00004774;Phosphonic Acid Bis(phenylmethyl) Ester;UNII-1O720L5H5A;1O720L5H5A;EINECS 241-226-1;Dibenzylphosphit;C14H15O3P;dibenzylphosphonate;SCHEMBL36370;Bis(benzyloxy)(oxo)phosphanium;DIBENZYL PHOSPHITE [MI];DTXSID20938038;MIBXHGZAARWAGI-UHFFFAOYSA-N;Dibenzyl phosphite, technical grade;C14-H15-O3-P;AKOS015964514;CS-W010143;s11959;AS-57330;SY010379;P1016;EN300-80532;Q-200957;Q27252680


 Xi
Xi


