10.1016/S0022-328X(00)00748-8
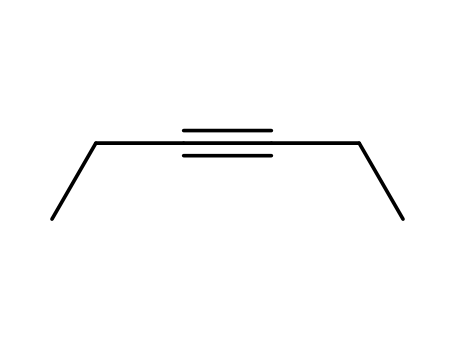
The research focuses on the synthesis and diastereoselective benzannulation of glucal-derived carbene complexes involving organotransition-metal-modified sugars. The study utilizes stannylated precursors 5 and 6 to prepare triisopropylsilyl and isopropylidene-protected 1-lithio-D-glucals, which react with hexacarbonyl chromium and subsequent methylation to yield D-arabino-hex-1-enopyranosylcarbene complexes 7 and 8. These complexes then undergo diastereoselective benzannulation with tolan and 3-hexyne to produce polyoxygenated chromans 9 to 12. The research emphasizes the role of protective groups in controlling the conformation of the glucal moiety in both the carbene ligand and the chroman skeleton. The study employs 1H-NMR studies and single crystal X-ray analyses to determine the conformations of the sugar moiety in solution and solid state, indicating a 5H4-conformation for triisopropylsilyl compounds and a 4H5-conformation for isopropylidene derivatives. The reactions and analyses involve various reagents, protective groups, and spectroscopic techniques, with a particular focus on the stereochemistry and conformational control in the synthesis of these complex organic molecules.
10.1021/jo00190a015
Chen-Chih Cheng, Frederick D. Greene, and John F. Blount investigates the reaction products and kinetics of 4-phenyl-1,2,4-triazolinedione (PhTAD) with various acetylenes, including 3-hexyne, cyclooctyne, and diarylacetylenes. The study reveals that the major product from reactions of PhTAD with diarylacetylenes is a 2:1 adduct, identified as a bis(azomethine imine) and confirmed by X-ray analysis. The kinetics show that the reaction is first order in both acetylene and PhTAD, suggesting a pathway involving an initial 1:1 adduct. The research also compares the reactivity of PhTAD with bromine, noting significant differences in the sensitivity to substituents on the unsaturated substrates, particularly for aryl-substituted systems. The findings provide insights into the mechanism of electrophilic addition reactions involving triazolinediones and acetylenes.


 F;
F;  Xn;
Xn;  Xi
Xi


