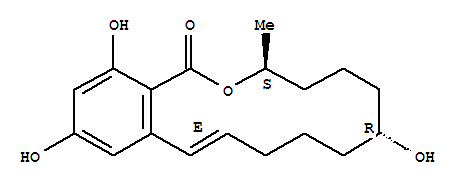
- Chemical Name:alpha-Zearalenol
- CAS No.:36455-72-8
- Molecular Formula:C18H24 O5
- Molecular Weight:320.386
- Hs Code.:29322090
- European Community (EC) Number:828-728-1
- UNII:59D4EVJ5KC
- DSSTox Substance ID:DTXSID8022402
- Nikkaji Number:J3.201.624C
- Wikipedia:Alpha-Zearalenol
- Wikidata:Q27890390
- Metabolomics Workbench ID:49563
- ChEMBL ID:CHEMBL371463
- Mol file:36455-72-8.mol
Synonyms:(-)-beta-zearalenol;(3R,7R,11E)-7,14,16-trihydroxy-3-methyl-3,4,5,6,7,8,9,10-octahydro-1H-2-benzoxacyclotetradecin-1-one;1H-2-benzoxacyclotetradecin-1-one, 3,4,5,6,7,8,9,10-octahydro-7,14,16-trihydroxy-3-methyl-, (3S,7R,11E)-;3,4,5,6,7,8,9,10-octahydro-7,14,16-trihydroxy-3-methyl-1H-2-benzoxacyclotetradecin-1-one;alpha-zearalenol;alpha-zearalenol, (cis)-isomer;beta-trans-zearalenol;beta-zearalenol;zearalenol



 Xn
Xn


