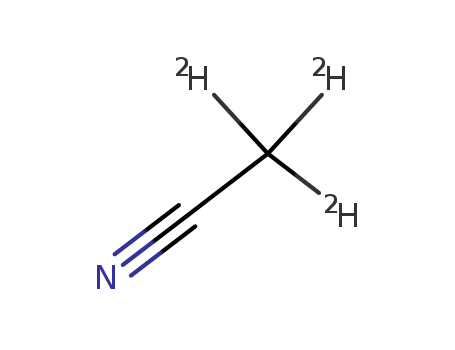
- Chemical Name:Acetonitrile-d3
- CAS No.:2206-26-0
- Molecular Formula:C2D3N
- Molecular Weight:44.0287
- Hs Code.:2845 90 10
- European Community (EC) Number:218-616-5
- DSSTox Substance ID:DTXSID50176547
- Nikkaji Number:J299.157H
- Wikidata:Q13640572
- Mol file:2206-26-0.mol
Synonyms:Acetonitrile-d3;2206-26-0;2,2,2-trideuterioacetonitrile;Methyl-d3 cyanide;(2H3)Acetonitrile;CD3CN;Acetonitrile D3;Acetonitrile-2,2,2-d3;Trideuteroacetonitrile;MFCD00001881;Acetonitrile-d3, 99.8 atom % D;acetonitril-d3;Acetonitrile-d3-;Acetonitrile D3 >99.8%;DTXSID50176547;CHEBI:193038;EINECS 218-616-5;AKOS015904206;Acetonitrile-d3, >=99.8 atom % D;Acetonitrile-d3, anhydrous, 99.8 atom % D;D88498;Acetonitrile-d3, "100%", 99.96 atom % D;Acetonitrile-d3, >=99.8 atom % D, anhydrous;J-506713;Acetonitrile-D3 (D, 99.8%) +0.03% V/V TMS;Acetonitrile-d3, "Special HOH", >=99.8 atom % D;Acetonitrile-d3, 96-97 atom % D, D2O 15-20 %;Q13640572;Acetonitrile-d3, 99.8 atom % D, contains 1 % (v/v) TMS;Acetonitrile-d3, 99.8 atom % D, contains 0.03 % (v/v) TMS;Acetonitrile-d3, 99.8 atom % D, contains 0.05 % (v/v) TMS


 T;
T;  F;
F;  N
N

