10.1007/s10593-009-0199-5
The research investigates the synthesis of novel tricyclic 2H,7H-furo[3',4':6,7]cyclohepta[1,2-b]pyran systems. The study aims to explore the reactions of 8-hydroxy-1,3-dimethyl-4H-cyclohepta[c]furan-4-one (1) with electrophilic alkenes containing good leaving groups, such as ethoxymethylenemalononitrile and ethoxymethylenecyanoacetate, in the presence of KOH. Contrary to expectations, these reactions produced noncyclic cyanovinyl derivatives as potassium salts (3a and 3b) rather than α-pyrones. However, these derivatives could be cyclized to the target α-pyrones (5a and 5b) under acid-catalyzed conditions. The study also synthesized 3-benzamido-2H-pyran-2-ones (9a-c) from 2-aryl-4-ethoxymethylene-4H-1,3-oxazol-5-ones using a similar scheme. The synthesized compounds, which may possess useful physiological activity, were characterized using spectroscopic methods. The research concludes that hydroxytropone 1 exhibits high nucleophilic reactivity with these electrophiles, leading to the formation of novel condensed α-pyrones and 3-benzamido-2H-pyran-2-ones through base-catalyzed and acid-catalyzed steps.
10.1021/jm00019a019
This research study on the synthesis and anticonvulsant activity of N-benzylpyrrolo[2,3-d]-, pyrazolo[3,4-d]-, and triazolo[4,5-d]pyrimidines, which are imidazole ring-modified analogues of 9-(2-fluorobenzyl)-6-(methylamino)-9H-purine (compound 1). The purpose of the study was to evaluate the impact of isosteric replacements of the imidazole ring atoms on the anticonvulsant activity of these compounds. The analogues were found to be less active than compound 1 against maximal electroshock-induced seizures (MES) in rats when administered orally. The research concluded that the differences in anti-MES activity were not due to variations in pKa or lipophilicity but could be related to the distinct electrostatic isopotential maps of the heterocyclic compounds, suggesting that the electrostatic potential on the surface of these compounds might influence their anticonvulsant activity. Key chemicals used in the synthesis process included various benzyl chlorides, amines, and pyrimidines, as well as reagents like potassium carbonate, ethoxymethylenemalononitrile, and formamide.
10.1021/jo01060a018
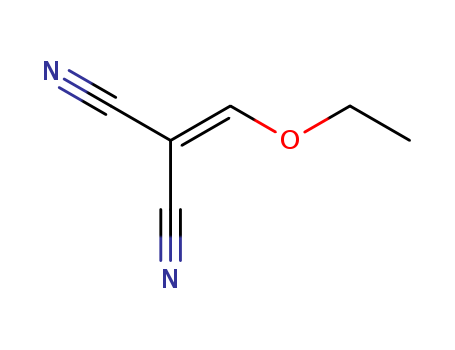
The study investigates the reactions of ethoxymethylenemalononitrile with various chemicals, primarily focusing on its behavior with thioureas in alkaline aqueous solutions. Ethoxymethylenemalononitrile can hydrolyze to form different compounds like hydroxymethylenemalononitrile, malononitrile, or tetracyanopropene, depending on the conditions. When reacted with thioureas, it forms 2-substituted-4-amino-5-cyanopyrimidines if added to the thiourea, and 2-alkylthio-4-amino-5-cyanopyrimidines in the presence of mercaptans or alkylthiour eas. The study also explores the formation of pyridines and their derivatives under various conditions, including the use of different solvents and the order of reagent addition. The products' structures are confirmed through synthesis and analysis, highlighting the versatility of ethoxymethylenemalononitrile in forming diverse nitrogen-containing heterocyclic compounds.


 Xn,
Xn, Xi,
Xi, T
T


