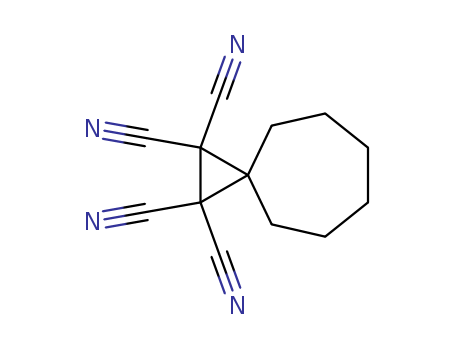
- Chemical Name:Spiro(2.6)nonane-1,1,2,2-tetracarbonitrile
- CAS No.:93086-95-4
- Molecular Formula:C13H12N4
- Molecular Weight:224.265
- Hs Code.:
- NSC Number:98481
- UNII:4LTV4EUB33
- DSSTox Substance ID:DTXSID60239258
- Nikkaji Number:J1.322.084K
- Wikidata:Q83121594
- ChEMBL ID:CHEMBL1980403
- Mol file:93086-95-4.mol
Synonyms:Spiro(2.6)nonane-1,1,2,2-tetracarbonitrile;93086-95-4;4LTV4EUB33;Spiro[2.6]nonane-1,1,2,2-tetracarbonitrile;NSC98481;NSC 98481;UNII-4LTV4EUB33;NSC-98481;tetracyanospiro[2.6]nonane;CHEMBL1980403;DTXSID60239258;NCI60_042197